S0845039
Flow splitter,for oxygen concentrator
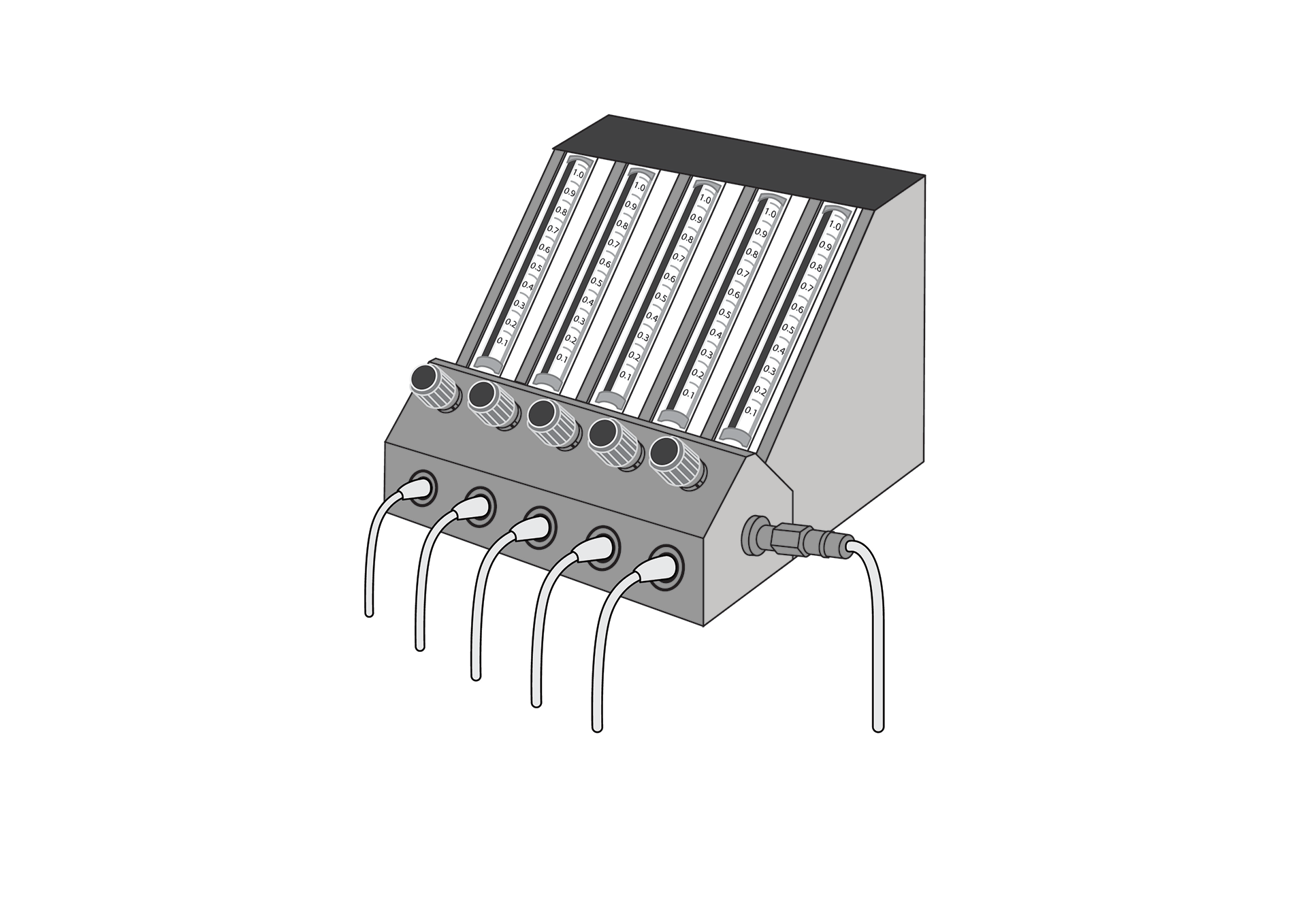
The oxygen flow splitter is a device intended to distribute medical oxygen from a source to multiple independent outlets. It can be connected to any low-pressure oxygen source, including concentrators, cylinders and a centralized system, and is suitable for table top or wall mounting.
Indicative Price 129.26 USD
GENERAL DESCRIPTION:
The oxygen flow splitter is a device intended to distribute medical oxygen from a source to multiple independent outlets. It can be connected to any low-pressure oxygen source, including concentrators, cylinders and a centralized system, and is suitable for tabletop or wall mounting.
TECHNICAL SPECIFICATION:
Equipped with five independent, Thorpe tube flow meters, to measure and regulate the flow of medical gas.
Suitable for cleaning and disinfection with hospital-grade cleaning products.
Inlet port is DISS format.
6 mm barbed outlets.
Flow meter columns are transparent, clearly readable and graduated (metric system).
Inlet pressure up to at least 20 psi (138 kPa).
0-2 L/min, accuracy better than 10%, graduation 0.125 L/min or lower.
SUPPLIED WITH
Connecting tube, 2m long, with DISS standard connectors each end for oxygen input.
User manual, to be supplied in local language, or agreed other language if local is not available
ITEMS REQUIRED, BUT NOT SUPPLIED:
Oxygen Concentrator
Humidifier
Nasal prongs
ENVIRONMENTAL CONDITIONS
Operating conditions: 5 to 40 deg C, relative humidity 15 to 90% non-condensing.
Storage conditions: 5 to 50 deg C, relative humidity 15 to 90% non-condensing.
WARRANTY:
24 months
REGULATION & CONFORMITY REQUIREMENTS
Accessory to oxygen concentrator with CE mark conforming to Medical Device Directive 93/42/EEC
Accessory to oxygen concentrator with CE Certificate (class IIa)
APPLICABLE STANDARDS
Complies with at least the following standards:
ISO 15001 Anaesthetic and respiratory equipment - Compatibility with oxygen
ISO 13485 Certificate for manufacturer’s QMS.
PACKAGING / LABELLING
Packing and labelling in compliance with the requirements as set out on the UNICEF Supply website under this hyperlink: https://www.unicef.org/s upply/technical-specifications-packing-packaging-and-labelling.
For medical devices: Any symbol used on the labelling is in accordance with the ISO 15223-1:2016 Medical devices - Symbols to be used with medical device labels, labelling and information to be supplied - Part 1: General requirements.
Related Products