S0845183
Cuffs, blood pressure, set 5 sizes
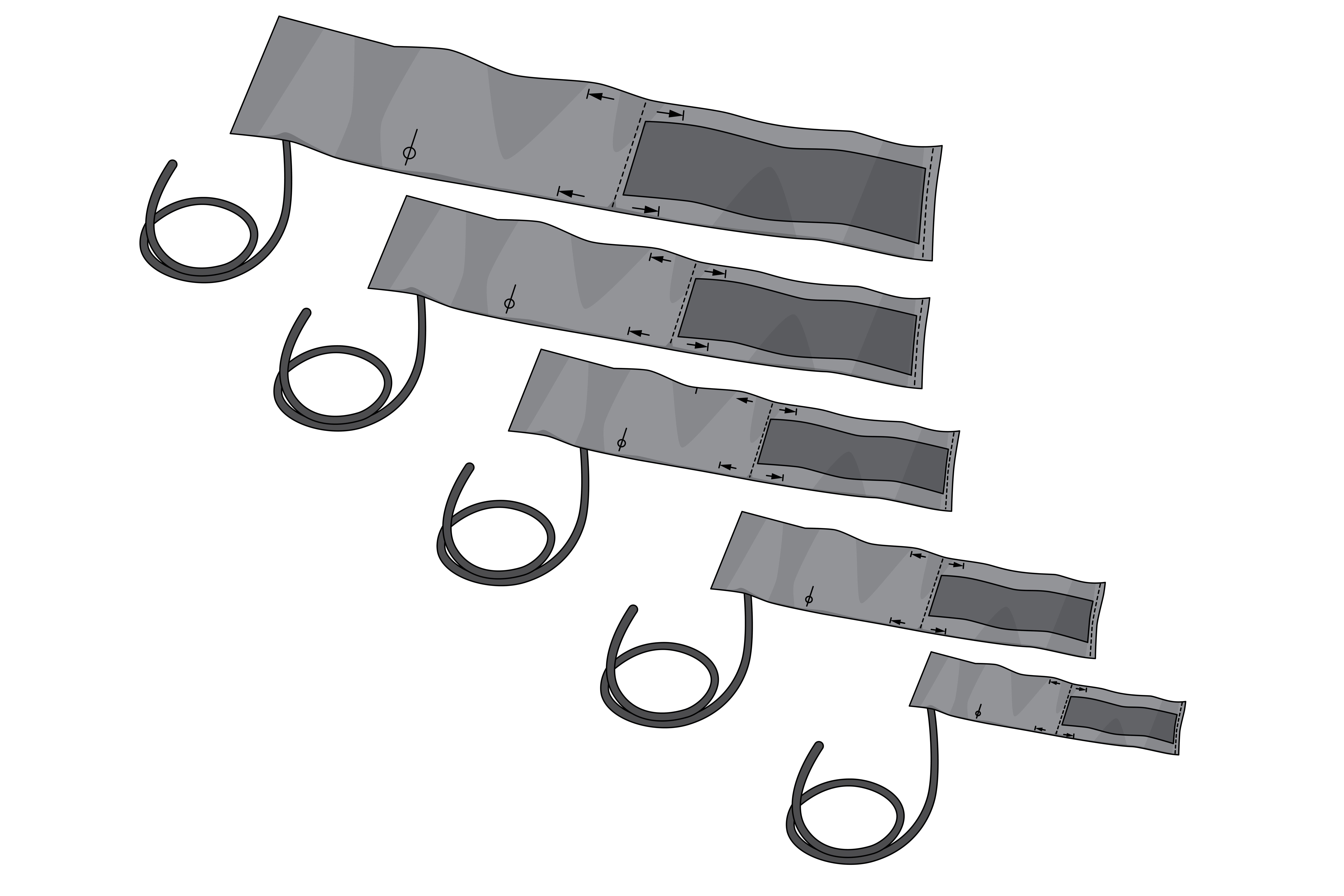
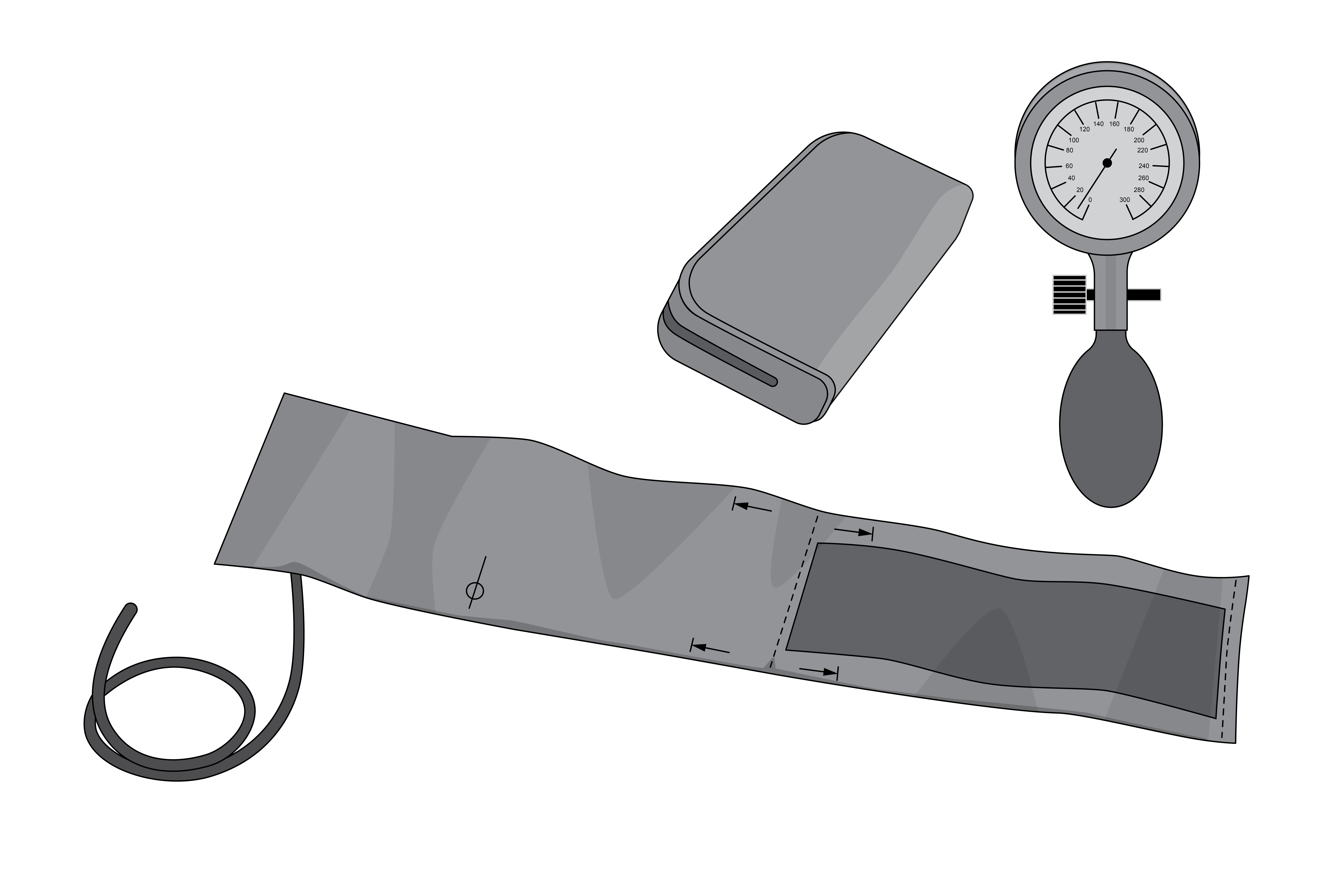
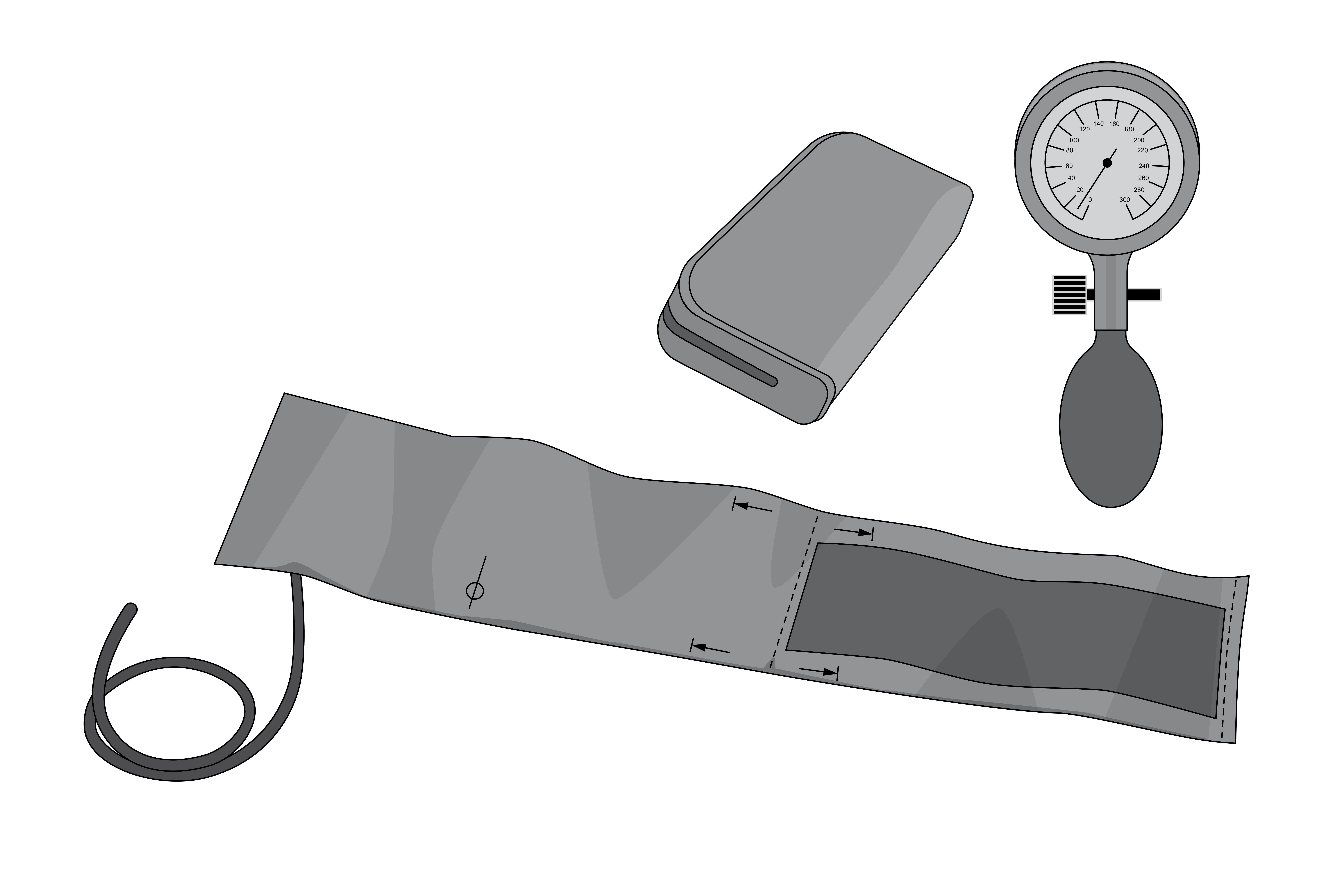
Cuff set for measuring blood pressure with manual sphygmomanometers. Comprises of a set of 5 reusable cuffs of different sizes: infant, child, small adult, adult, and large adult. This contains only the 5 cuffs and does not include the manometer or bulb required to carry out any measurements.
Indicative Price 17.72 USD
GENERAL DESCRIPTION
Set of 5 different sizes cuffs to be used with an existing blood pressure meter.
INTENDED USE
A band-like device that has an inflatable bladder in an inelastic sleeve with one or two connecting tubes with connector (typically locking connectors) that can be connected to a mechanism for inflating and deflating the bladder and/or a sphygmomanometer or a patient monitoring device/system that is used to determine a patient's blood pressure. It is wrapped around the upper arm or the leg (thigh) of the patient. This is a reusable device which may be intended for single-patient or multi-patient reuse.
TECHNICAL SPECIFICATIONS
Composed of a cuff containing an inflatable bag.
The inflatable bag has a single tube.
The cuff is made of durable material (e.g. nylon), which is non-deformable, and washable at 30°C.
The cuff is fitted with Velcro fastening, enabling a tight and secure fit around arms.
The cuff is reinforced at both sides.
Set of 5 sizes:
- Infant
- Child
- Small adult
- Adult
- Large adult
Material tube: rubber or other suitable material, e.g. silicone rubber, crack resistant.
Length tube between: 50 to 70 cm.
SUPPLIED WITH
Instructions for assembly, use and maintenance in English, French and Spanish.
SHELF LIFE
Not applicable.
ESTIMATED LIFE SPAN
Three to five years.
WARRANTY
One year.
ENVIRONMENTAL CONDITIONS
Storage conditions: -20 - 70°C / 85% RH.
Operating conditions: 10 - 40°C / 85% RH.
WEIGHT AND VOLUME (Packaged)
Weight: 400 gr.
Volume: 2.00 dm³.
INSTALLATION / COMMISSIONING REQUIREMENTS
There are no installation or commissioning requirements.
TRAINING REQUIREMENTS
Where staff has experience with these types of devices, no user training will be required.
MAINTENANCE/USER REQUIREMENTS
Other than cleaning in accordance with manufactures instructions there are not specific maintenance requirements.
RELATED PRODUCTS
S0683200, Sphygmomanometer,(adult),aneroid
S0845187, Sphygmomanometer,(adult),resilient
S0683300, Sphygmomanometer,(child),aneroid
S0845188, Sphygmomanometer,(child),resilient
COMPONENT OF A KIT
No.
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier is certified for ISO 9001 Quality management systems – Requirements.
MARKET CLEARANCE AND DEVICE CLASSIFICATION
CE certified under EU MDD 93/42 as a Class Im device.
NOMENCLATURE
GMDN Code: 34978
Related Products