S0845210
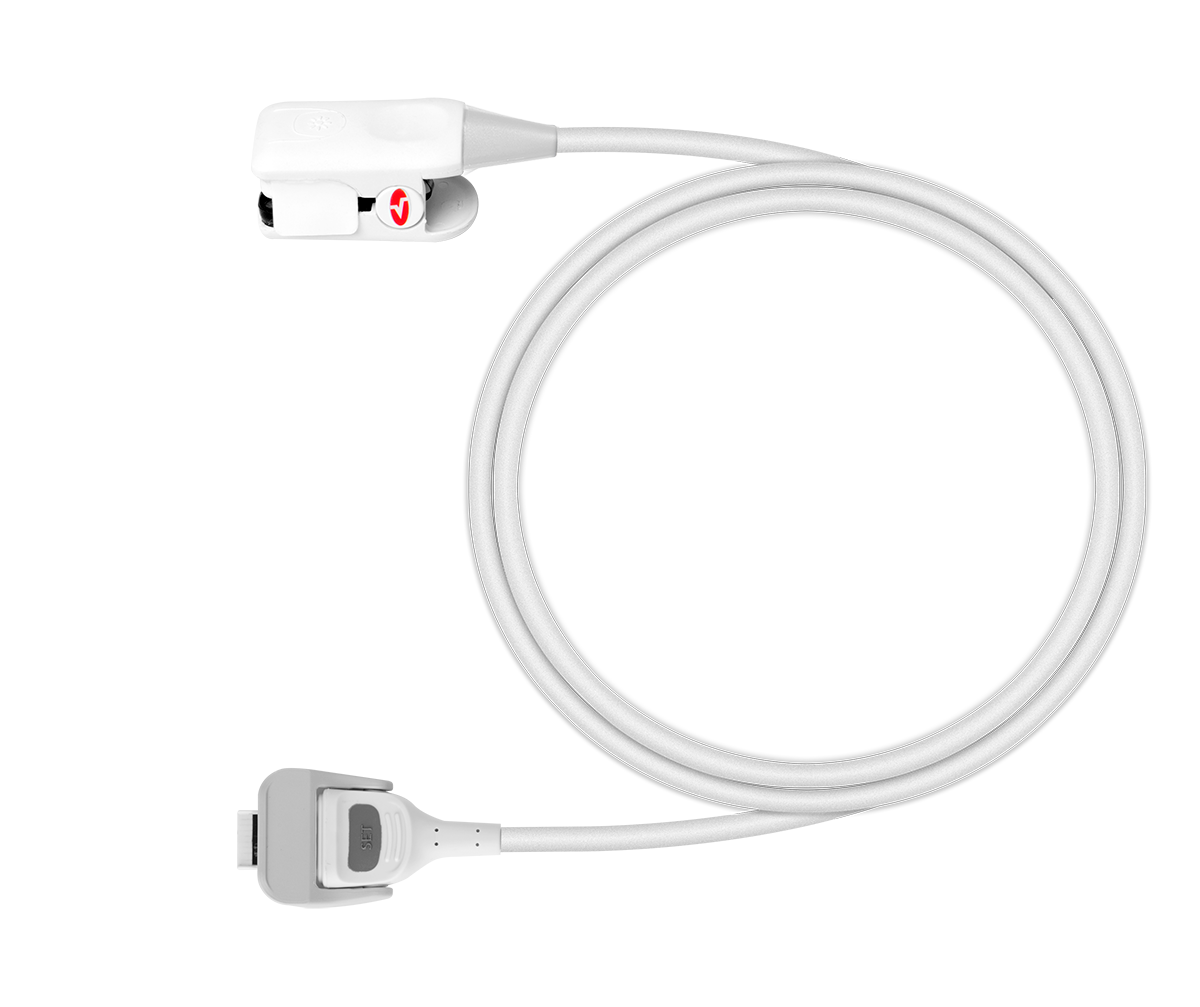
Sensor, reusable, Rad-G, child/adult
Reusable universal finger clip sensor for Masimo Rad-G™ handheld pulse oximeter, appropriate for use with adults, children and infants (≥ 3kg).
Indicative Price 120.00 USD
GENERAL DESCRIPTION
Reusable universal finger clip sensor for Masimo Rad-G™ handheld pulse oximeter, appropriate for use with adults, children and infants (≥ 3kg). Masimo reference PN 4325.
INTENDED USE
The Rad-G™ reusable sensors are indicated for noninvasive spot-checking of functional oxygen saturation of arterial hemoglobin (SpO2) and pulse rate (measured by an SpO2 sensor) for use with adult, pediatric, and infant (≥ 3 kg) patients during both no motion and motion conditions, and for patients who are well or poorly perfused in hospitals, hospital-type facilities, mobile, and home environments. They are not intended for long-term monitoring. They must be removed and repositioned to a different monitoring site at least every four (4) hours.
The Rad-G sensor is only compatible with the Rad-G device.
Prior to using this sensor, the user should read and understand the Operator’s Manual for the Rad-G device and the Directions for Use included with the reusable sensor.
ENVIRONMENTAL CONDITIONS
Storage Temperature: -40°C to +70°C.
Storage Humidity: 10% to 95% relative humidity (non-condensing).
SUPPLIED WITH
1 x Instructions for use in English/French/Spanish at minimum. Other languages can be requested. If digital manuals will be provided, then a quick reference guide in English/French/Spanish languages which clearly indicates the URL to access complete manuals will be supplied with each device.
WARRANTY
12-month warranty, dated from the completion of the terms of delivery.
WEIGHT & VOLUME (Packaged)
Weight: 0.1kg
Volume: 0.52dm³
ESTIMATED LEAD TIME
3 weeks
MARKET CLEARANCE AND DEVICE CLASSIFICATION
CE certified under the EU MDD 93/42/EEC or MDR 2017/745/EEC as Class IIb device.
QUALITY MANAGEMENT SYSTEM
Manufacturer is certified to ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
SAFETY AND PRODUCT STANDARDS
Complies with the following standards:
ISO 14971 Medical Devices -- Application of risk management to medical devices.
ISO 80601-2-61 Medical electrical equipment -- Part 2-61: Particular requirements for basic safety and essential performance of pulse oximeter equipment.
IEC 60601-1 Medical electrical equipment -- Part 1: General requirements for basic safety and essential performance.
IEC 60601-1-2 Medical electrical equipment -- Part 1-2: General requirements for basic safety and essential performance -- Collateral standard: Electromagnetic compatibility -- Requirements and tests.
Related Products