S0845017
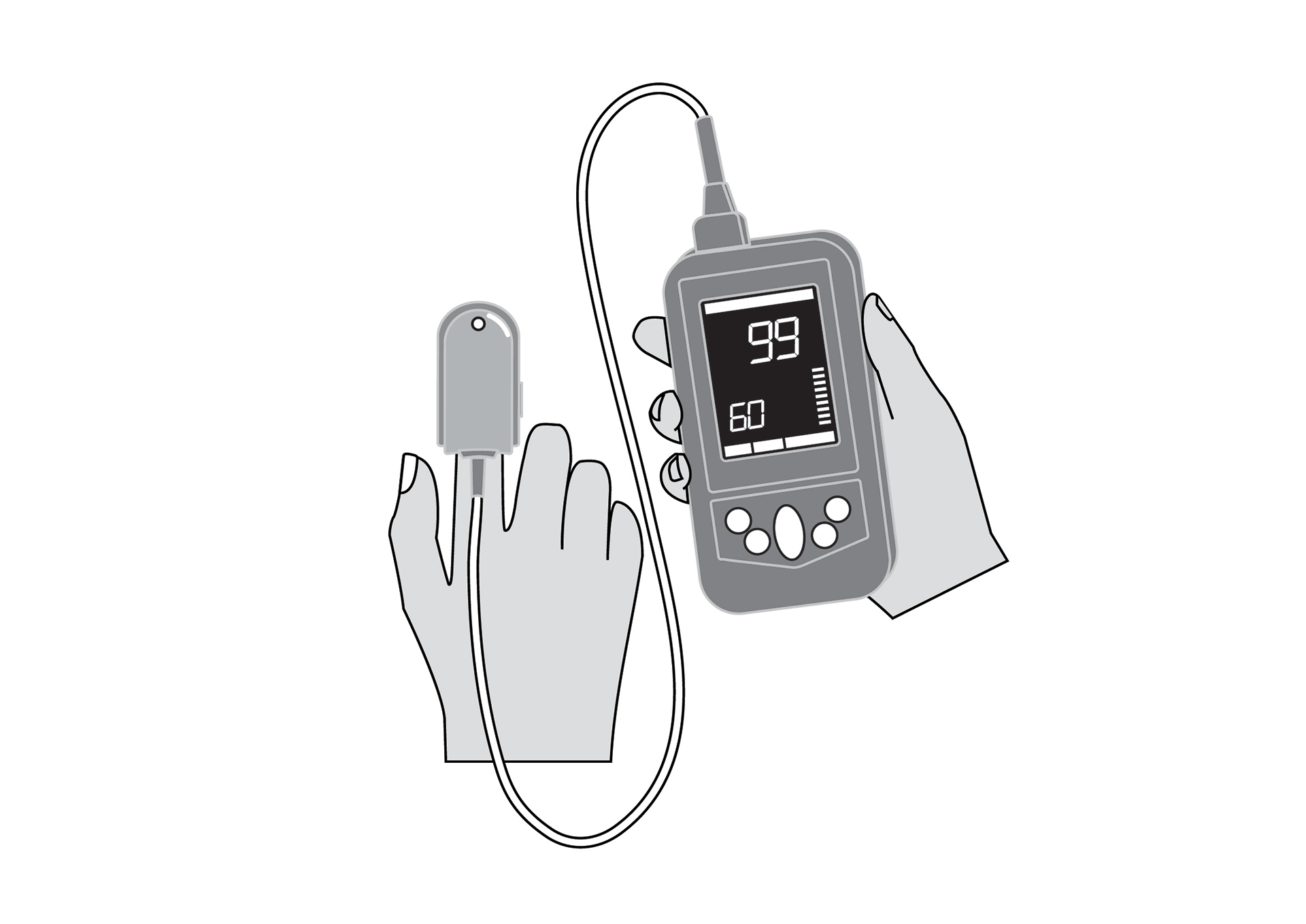
Pulse oximeter,handheld, incl.resp. rate
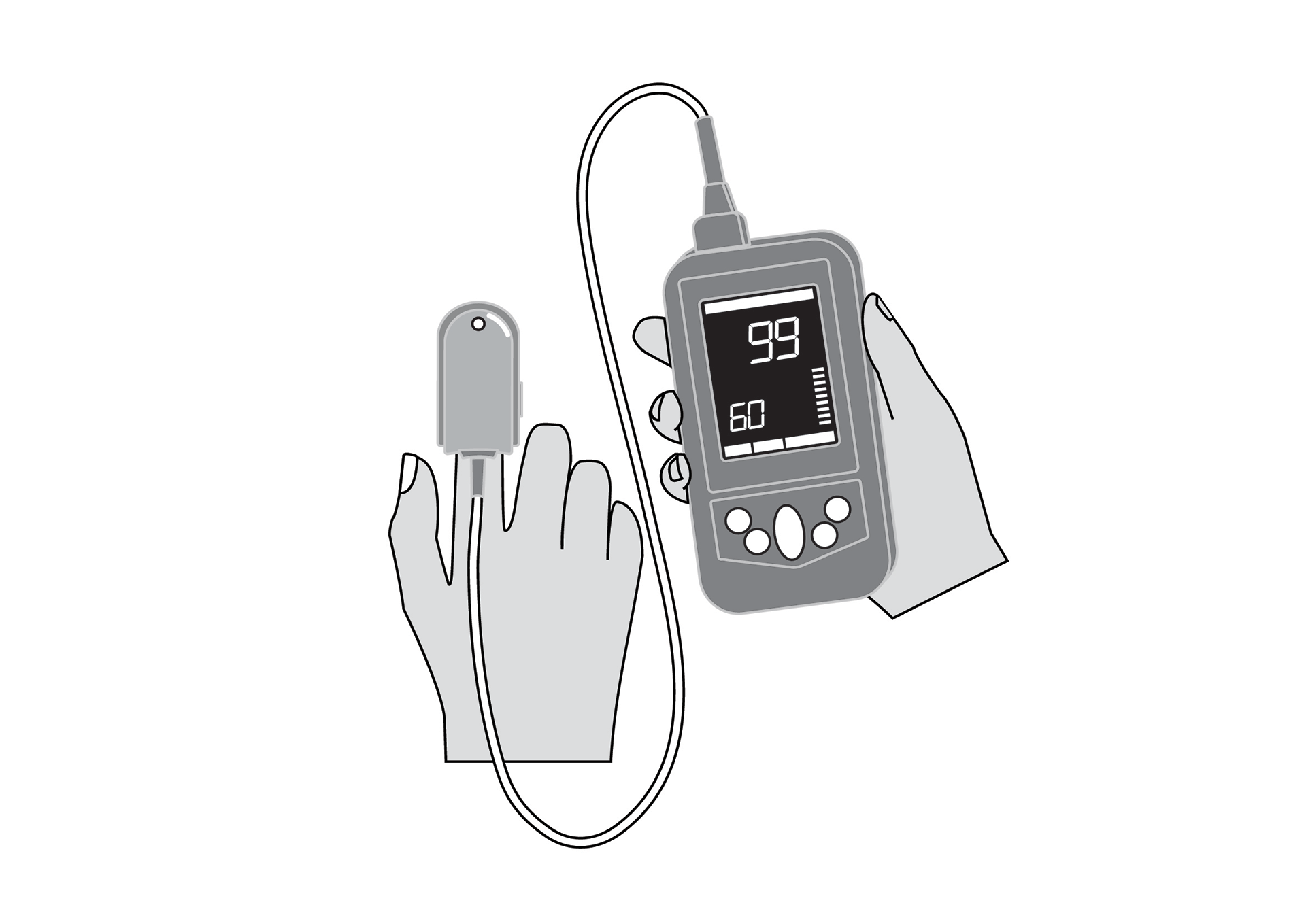
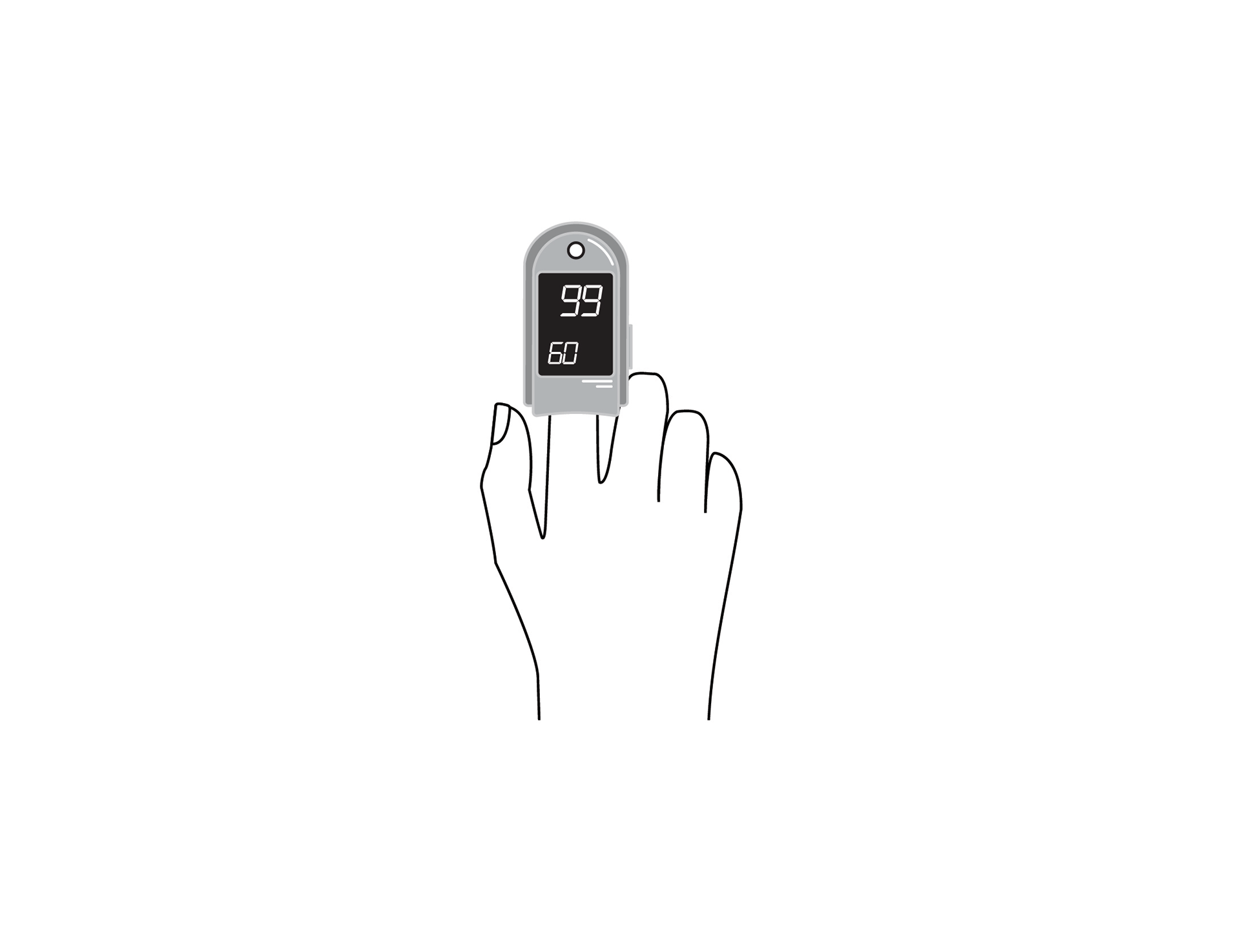
Handheld, portable, rechargeable battery-powered pulse oximeter, displaying patient oxygen saturation (SpO2), pulse rate and respiratory rate. It is intended for spot-checking and includes decision-assist features for pneumonia screening.
Indicative Price 370.00 USD
GENERAL DESCRIPTION
Handheld, portable, rechargeable battery-powered pulse oximeter, displaying patient oxygen saturation (SpO2), pulse rate and respiratory rate. It is intended for spot-checking and includes decision-assist features for pneumonia screening.
TECHNICAL SPECIFICATIONS
Measurement:
SpO2, pulse rate and respiratory rate monitor, with plethysmography waveform, for adults, children and neonates.
SpO2 detection includes the range: 70 - 100%.
SpO2 resolution: 1%.
SpO2 accuracy (in the range at least 70 - 100%): ± 2% (no motion, low perfusion), ± 3% (motion) for all patients (infants, children, adults).
Accuracy in patients with dark skin pigmentation has been validated through clinical studies.
Pulse Rate detection range: 25 - 240 bpm.
Pulse Rate resolution: 1 bpm.
Pulse Rate accuracy: within ± 3 bpm (no motion, low perfusion), ± 5 bpm (motion) for all patients (infants, children, adults).
Respiratory rate range: 4 to 90 rpm.
Respiratory rate resolution: 1 rpm.
Respiratory rate accuracy: ± 2 rpm.
Display:
Data display update rate: every 1s.
Display with main parameters: % SpO2, plethysmographic waveform, pulse rate, respiratory rate, signal quality, status messages, battery state indication.
Patient age group input feature.
Display indicates high and normal respiratory count for inputted age based off of WHO/IMCI guidelines.
Display indicates when SpO2 is < 90%.
Features:
Suitable for detection in low perfusion conditions.
Design enables use in demanding environments, e.g. shock, vibration.
Audible and visual alarms for sensor error or disconnected, system errors, low battery.
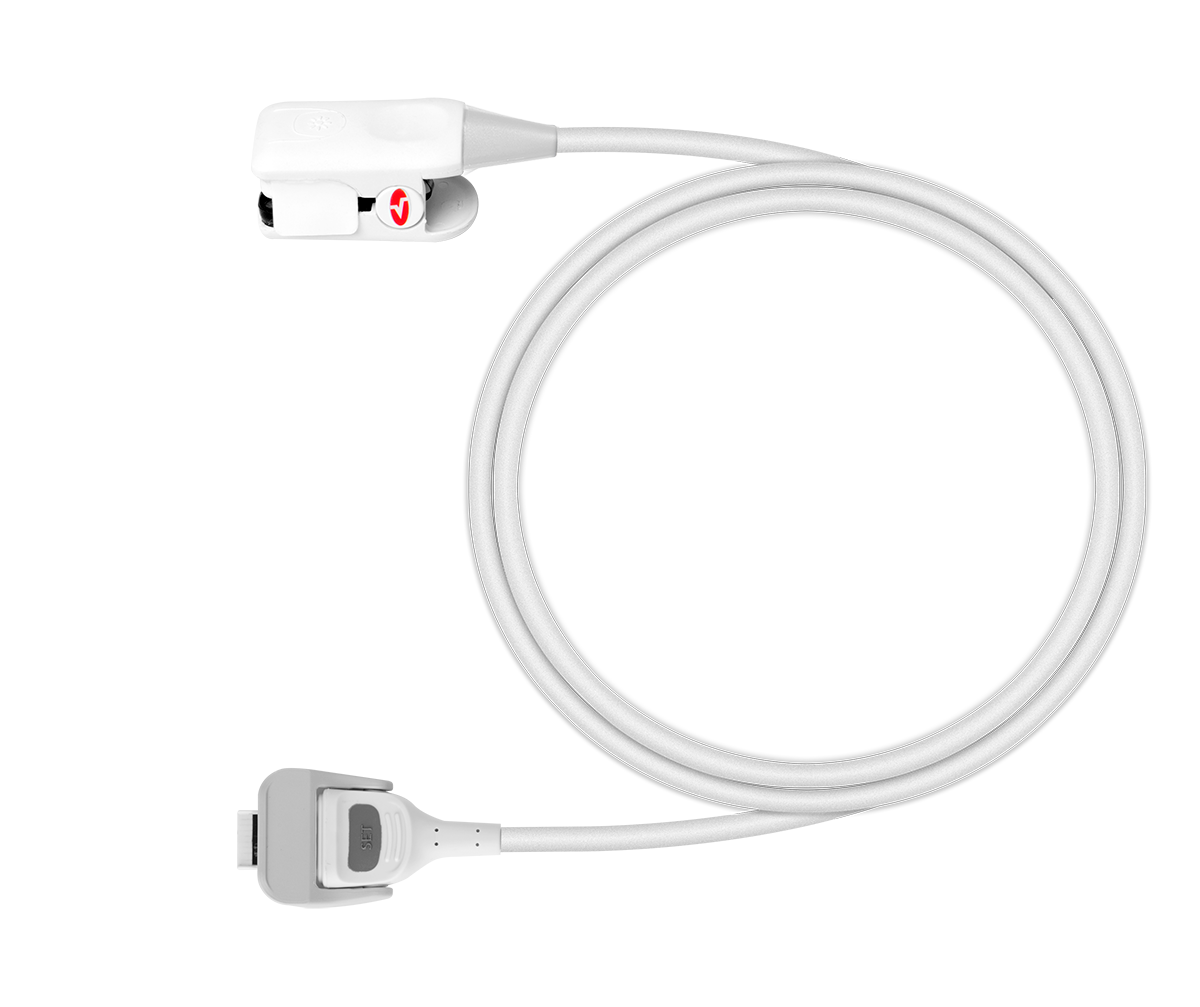
Capable of working with adult, paediatric and neonatal reusable probes.
Casing:
Enclosure protection IP22.
Case and reusable probe suitable for cleaning and disinfection.
Overall device and probe weight < 400 g.
Electrical characteristics:
Operated by rechargeable Li-ion battery power supply.
External AC battery charger, plug style and voltage as per local supply.
Charger protects against over-voltage and over-current line conditions and is certified to IEC 60601-1.
Protection against defibrillator discharges.
Suitable for operating by mains power and by battery.
Automatic switch between battery and mains powered modes.
The display shows which power source is in use.
Running time on battery ≥ 12 hours (24h at lowest screen brightness and beep tone turned off).
ENVIRONMENTAL CONDITIONS
Storage conditions (packaged): -20 to 60°C, 10 to 95% relative humidity, non-condensing.
Operating conditions: 0 to 40°C while battery is charging, 0 to 50°C while battery is not charging, 10 to 95% relative humidity, non-condensing.
SUPPLIED WITH
2 x universal reusable sensors (for adults or children ≥ 3kg)
1 x battery charger
1 x set user and maintenance manuals in English/French/Spanish at minimum. Other languages can be requested. If digital manuals will be provided, then a quick reference guide in English/French/Spanish languages which clearly indicates the URL to access complete manuals will be supplied with each device.
1 x list of all equipment and procedures required for routine maintenance in Operator’s Manual.
1 x list of all spares and accessories, with part numbers and contact details for part supply in Operator’s Manual.
1 x contact details of manufacturer, supplier and local service agent in Operator’s Manual.
ITEMS THAT MAY BE REQUIRED BUT ARE NOT INCLUDED
S0845211 Sensor, reusable, Rad-G, neonate, YI set
S0845209 Foam wraps, for YI sensor, Rad-G/PAC-12
WARRANTY
Includes 2-year extended warranty, dated from the completion of the terms of delivery.
AFTER SALES AND TRAINING SERVICES
For 1 to 4,999 pulse oximeters procured, supplier will provide at no additional cost:
-- Virtual training platform via a QR code
-- Remote service and support for lifetime of product
For a single purchase of 5,000 – 9,999 pulse oximeter units for a single country, supplier will provide at no additional cost:
-- Virtual training platform via a QR code
-- Remote service and support for lifetime of product
-- 1-year Protection+ Service; upgraded warranty to include non-manufacturer-related issues such as battery replacement, accidental damage, and performance verification.
-- Initial on-site training for first year
For a single purchase of 10,000 or more pulse oximeter units for a single country, supplier will provide at no additional cost:
-- Virtual training platform via a QR code
-- Remote service and support for lifetime of product
-- 1-year Protection+ Service; upgraded warranty to include non-manufacturer-related issues such as battery replacement, accidental damage, and performance verification.
-- Quarterly on-site training for first year
LIFESPAN
Estimated device service life: 10 years.
Supplier commits to the availability of spare parts & consumables for the service lifespan.
WEIGHT & VOLUME (Packaged)
Weight: 1.0kg
Volume: 4.3dm³
ESTIMATED LEAD TIME
3 weeks
MATERIAL SAFETY DATA SHEET INFORMATION (MSDS)
Li-Ion (Lithium-Ion) battery, UN 3481 Class 9, Packing Group II.
MARKET CLEARANCE AND DEVICE CLASSIFICATION
CE certified under the EU MDD 93/42/EEC or MDR 2017/745/EEC as Class IIb device.
QUALITY MANAGEMENT SYSTEM
Manufacturer is certified to ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
SAFETY AND PRODUCT STANDARDS
Complies with the following standards:
ISO 14971 Medical Devices -- Application of risk management to medical devices.
ISO 80601-2-61 Medical electrical equipment -- Part 2-61: Particular requirements for basic safety and essential performance of pulse oximeter equipment.
IEC 60601-1 Medical electrical equipment -- Part 1: General requirements for basic safety and essential performance.
IEC 60601-1-2 Medical electrical equipment -- Part 1-2: General requirements for basic safety and essential performance -- Collateral standard: Electromagnetic compatibility -- Requirements and tests.
Related Products