S0845221
Pulse oximeter, fingertip, class IIb
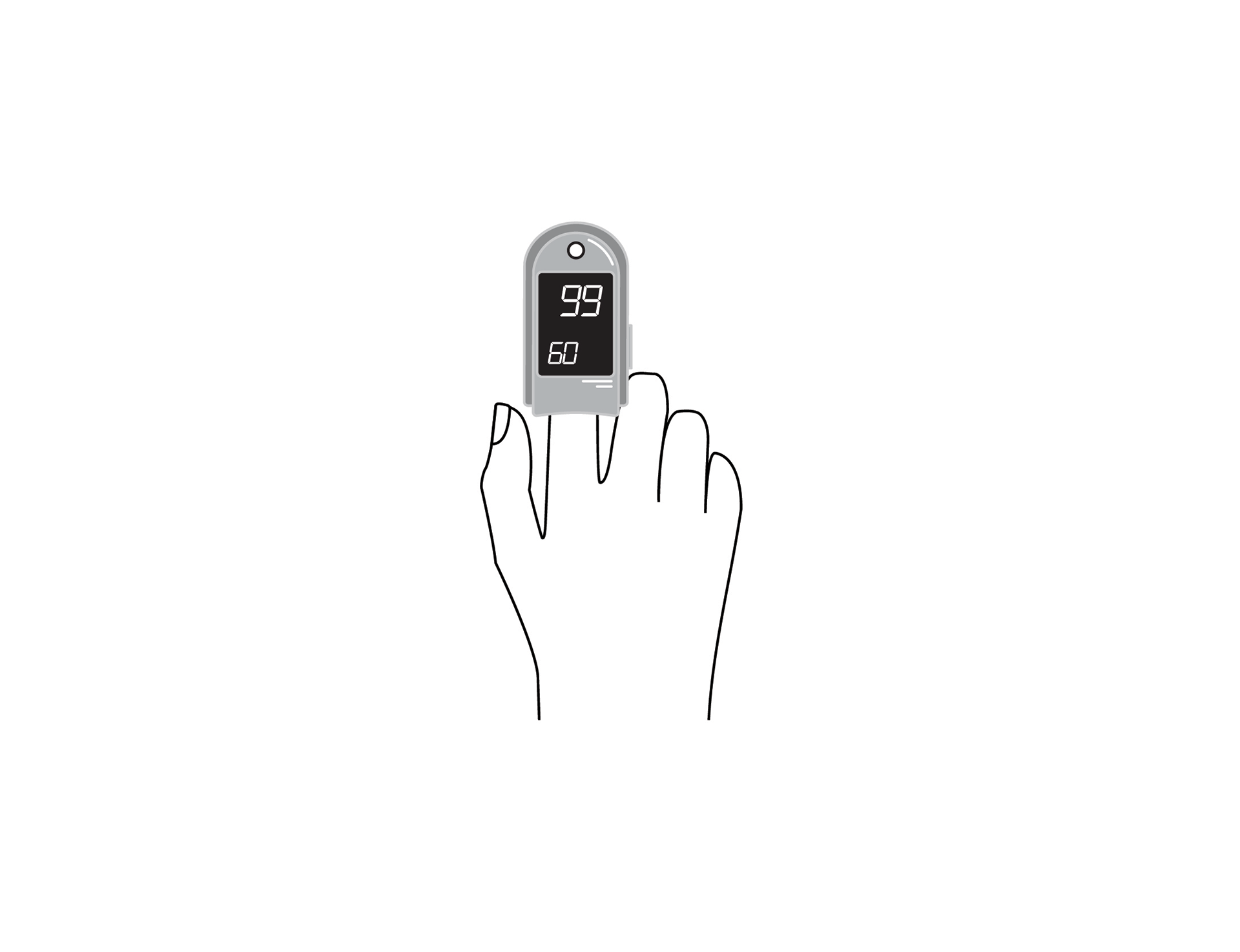
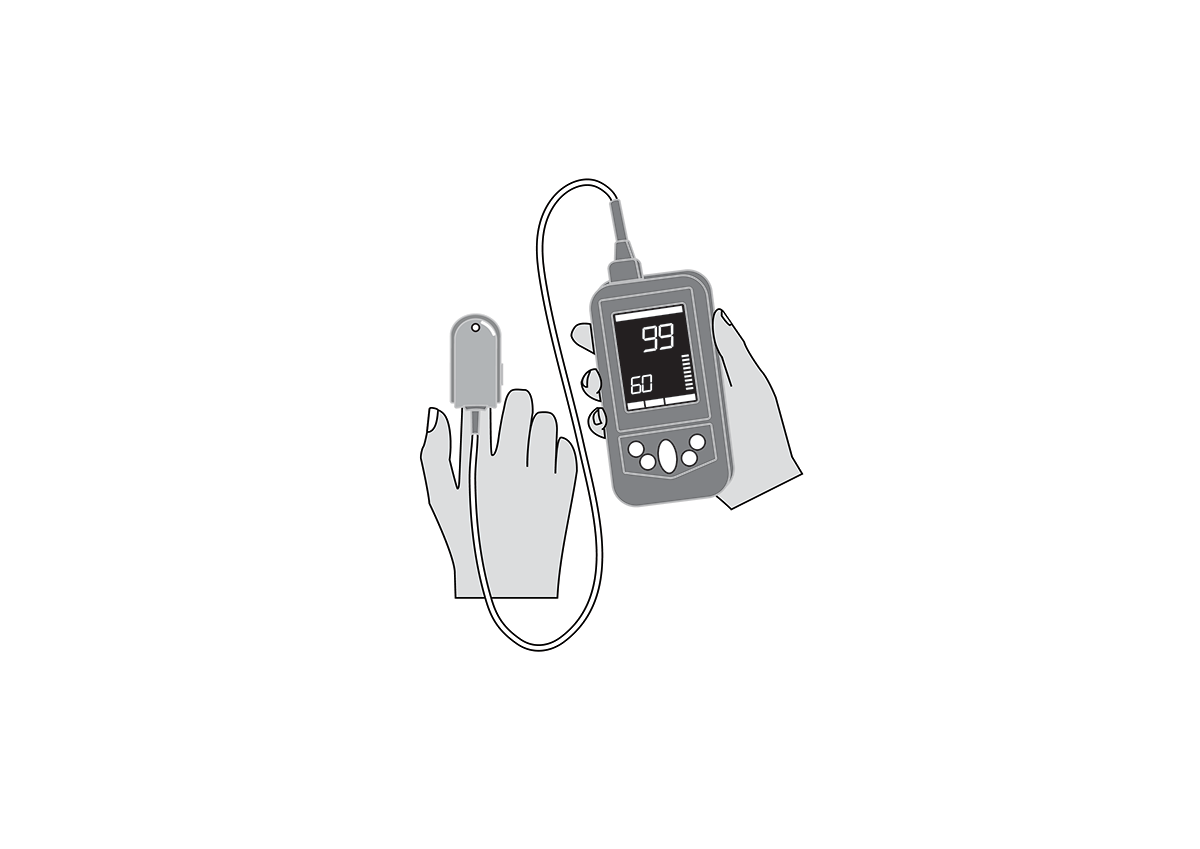
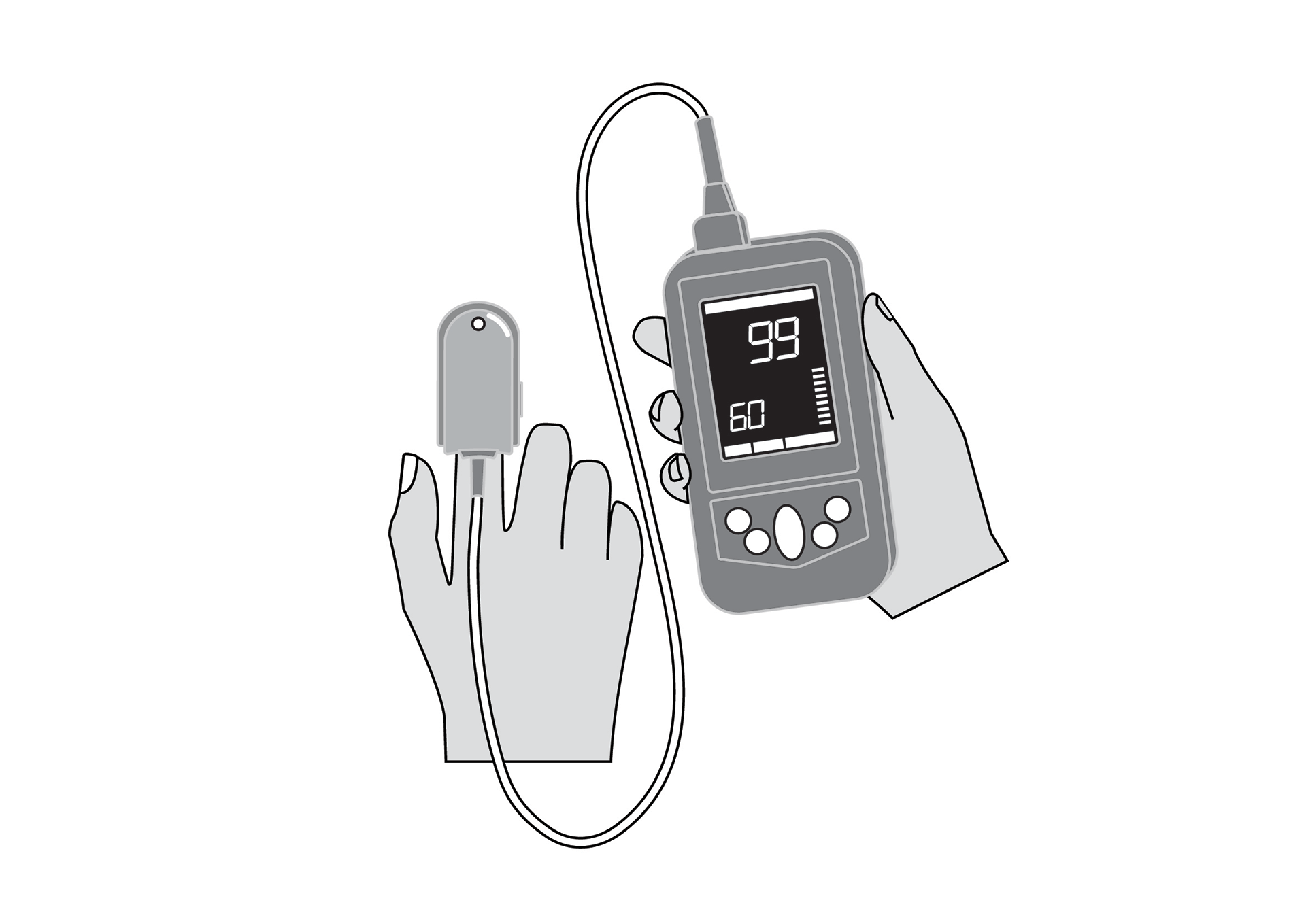
Finger clip mounted, battery-powered, all-in-one pulse oximeter displaying patient oxygen saturation (SpO2) and pulse rate. Intended for spot-checking of adult and paediatric patients.
Indicative Price 153.55 USD
GENERAL DESCRIPTION
Finger clip mounted, battery-powered, all-in-one pulse oximeter displaying patient oxygen saturation (SpO2) and pulse rate. It is intended for spot-checking of adult and paediatric patients. EU medical device risk classification: IIb.
INTENDED USE ENVIRONMENTS
Hospitals, clinics, long-term care facilities, skilled nursing facilities, emergency medical services and home healthcare services.
TECHNICAL SPECIFICATIONS
Measurement:
SpO2 and pulse rate monitor for adults and children.
Specified for finger thickness from 8mm to 25mm.
SpO2 detection includes the range: 0 - 100%.
SpO2 resolution: 1%.
SpO2 accuracy in the range 70 - 100%: ± 3% or better in normal, motion and low perfusion conditions for all patients (children, adults).
Accuracy in patients with dark skin pigmentation has been validated through clinical studies.
Pulse Rate detection includes the range 20 - 250 bpm.
Pulse Rate resolution: 1 bpm.
Pulse Rate accuracy ± 3 bpm.
Display:
Display shows % SpO2, pulse rate, signal quality, sensor error and low battery.
Features:
Design enables use in demanding environments e.g. shock, vibration as per tests in ISO 80601-2-61.
Capable of withstanding a minimum of 50 drops.
Enclosure protection IP33.
Suitable for cleaning and disinfection with hospital-grade cleaning products.
Electrical characteristics:
Operated by 2 x AAA alkaline batteries.
Batteries allow approximately 6,000 spot checks or 36 hours of continuous operation.
Automatic power-off.
ENVIRONMENTAL CONDITIONS
Storage conditions: -40 to 70°C, 10 to 95% RH.
Operating conditions: -5 to 40°C, 10 to 90% RH.
SUPPLIED WITH
2 x sets of single-use AAA batteries (i.e. 4 x AAA batteries in total).
1 x neck lanyard for carrying.
1 x carry/storage case.
1 x set user and maintenance manuals in English/French/Spanish at minimum. Other languages can be requested. If digital manuals will be provided, then a quick reference guide in English/French/Spanish languages which clearly indicates the URL to access complete manuals will be supplied with each device.
1 x list of all equipment and procedures required for routine maintenance.
1 x contact details of manufacturer in IFU.
WARRANTY
Includes 4-year standard warranty, dated from the completion of the terms of delivery.
ESTIMATED LIFE SPAN
5 years.
WEIGHT & VOLUME
Weight: 0.195kg
Volume: 1.89dm³
MATERIAL SAFETY DATA SHEET INFORMATION (MSDS)
Alkaline battery.
MARKET CLEARANCE AND DEVICE CLASSIFICATION
CE certified under the EU MDD 93/42/EEC or MDR 2017/745/EEC as Class IIb device.
USA FDA (Class II).
QUALITY MANAGEMENT SYSTEM
Manufacturer is certified to ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
SAFETY AND PRODUCT STANDARDS
Complies with the following standards:
ISO 14971 Medical Devices - Application of risk management to medical devices.
ISO 80601-2-61 Medical electrical equipment - Part 2-61: Particular requirements for basic safety and essential performance of pulse oximeter equipment.
IEC 60601-1 Medical electrical equipment - Part 1: General requirements for basic safety and essential performance.
IEC 60601-1-2 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests.
Related Products