S0845014
Pulse oximeter,portable,w/access
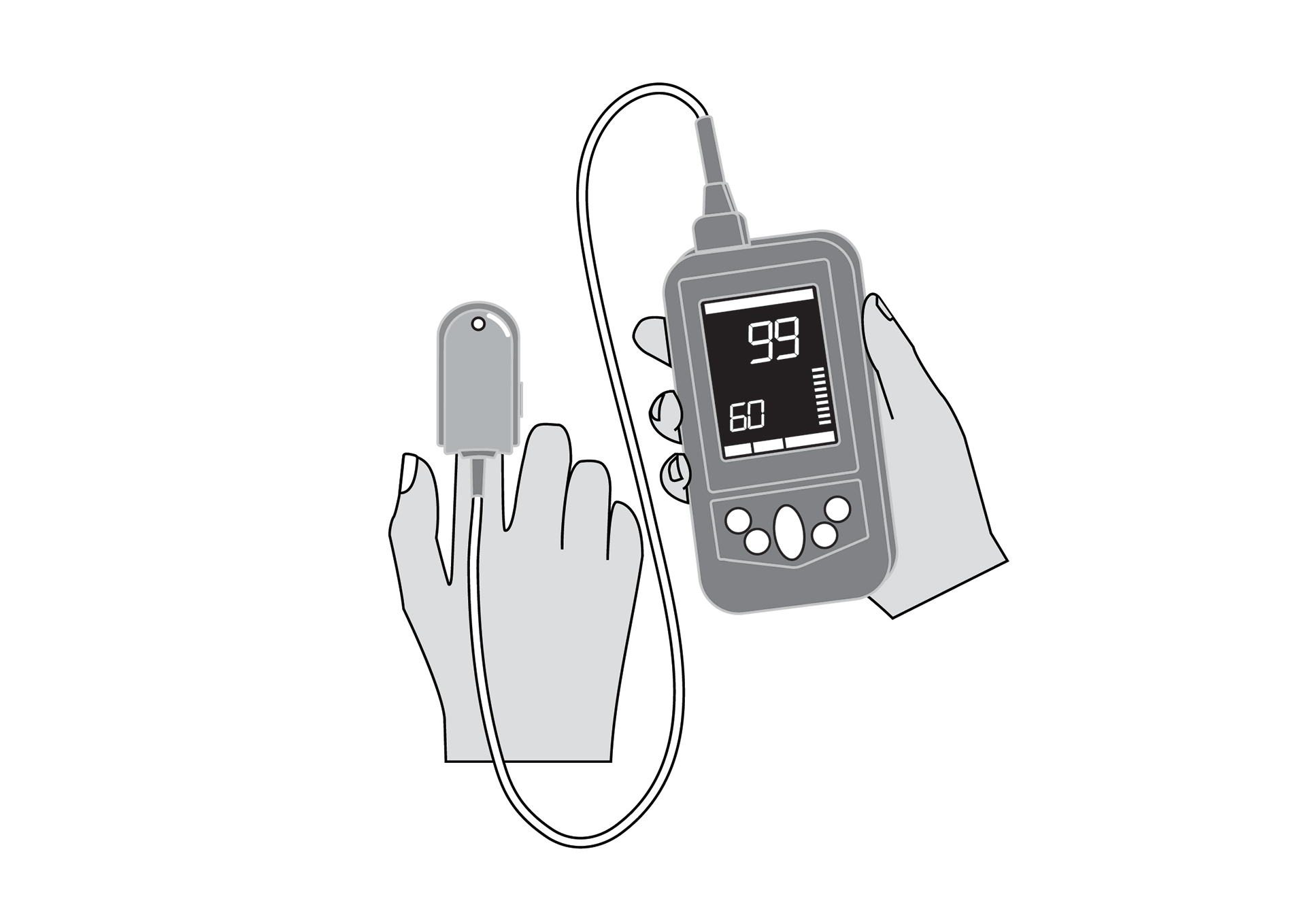
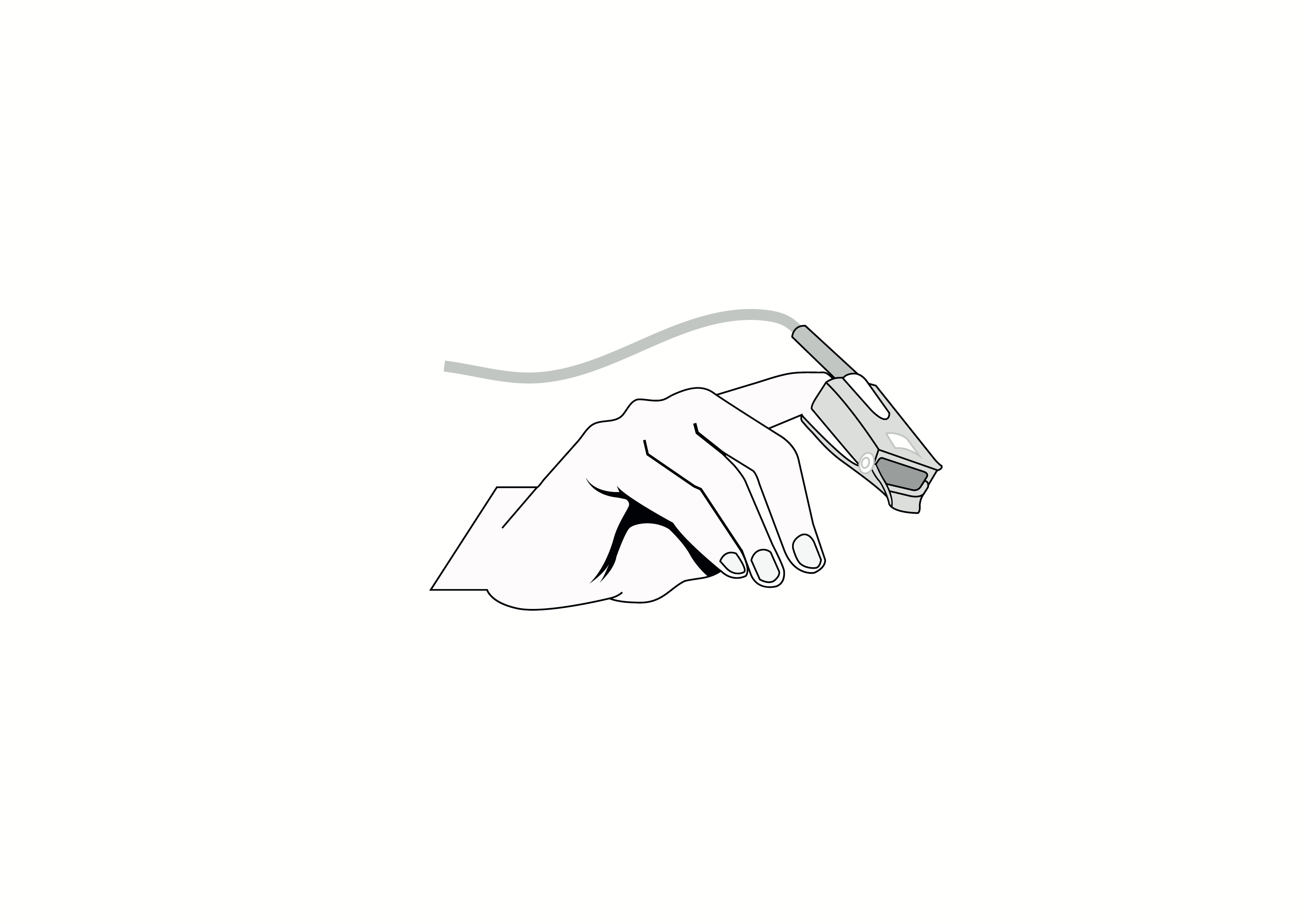
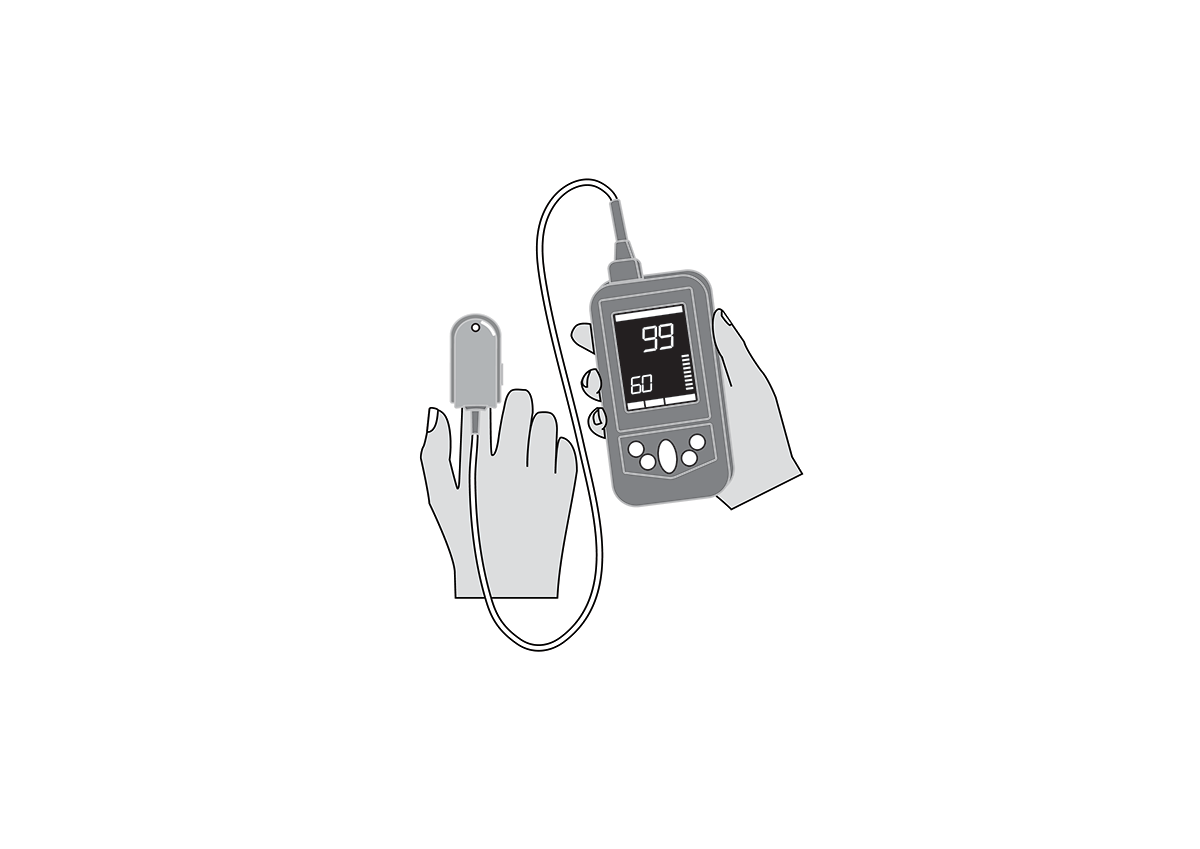
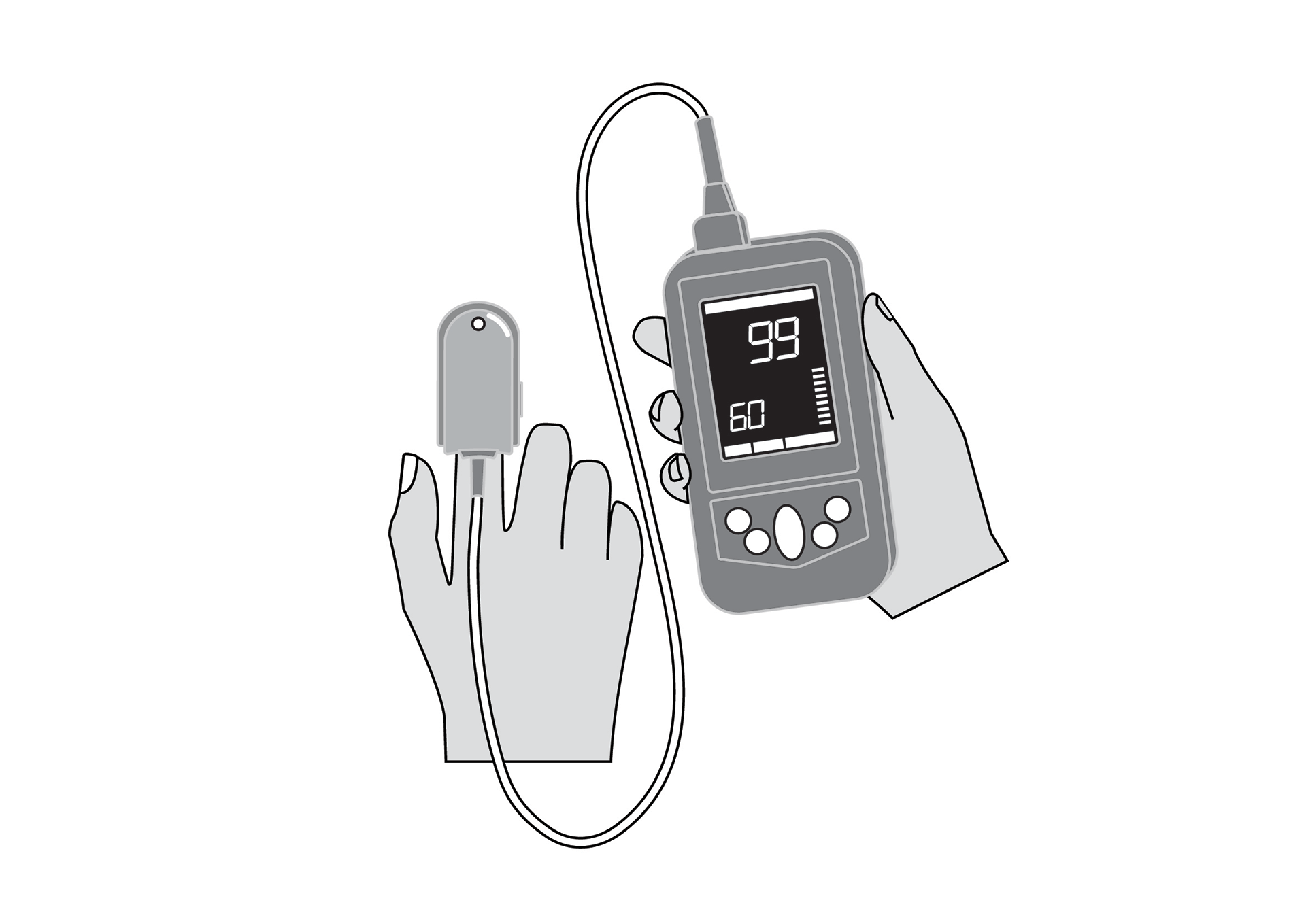
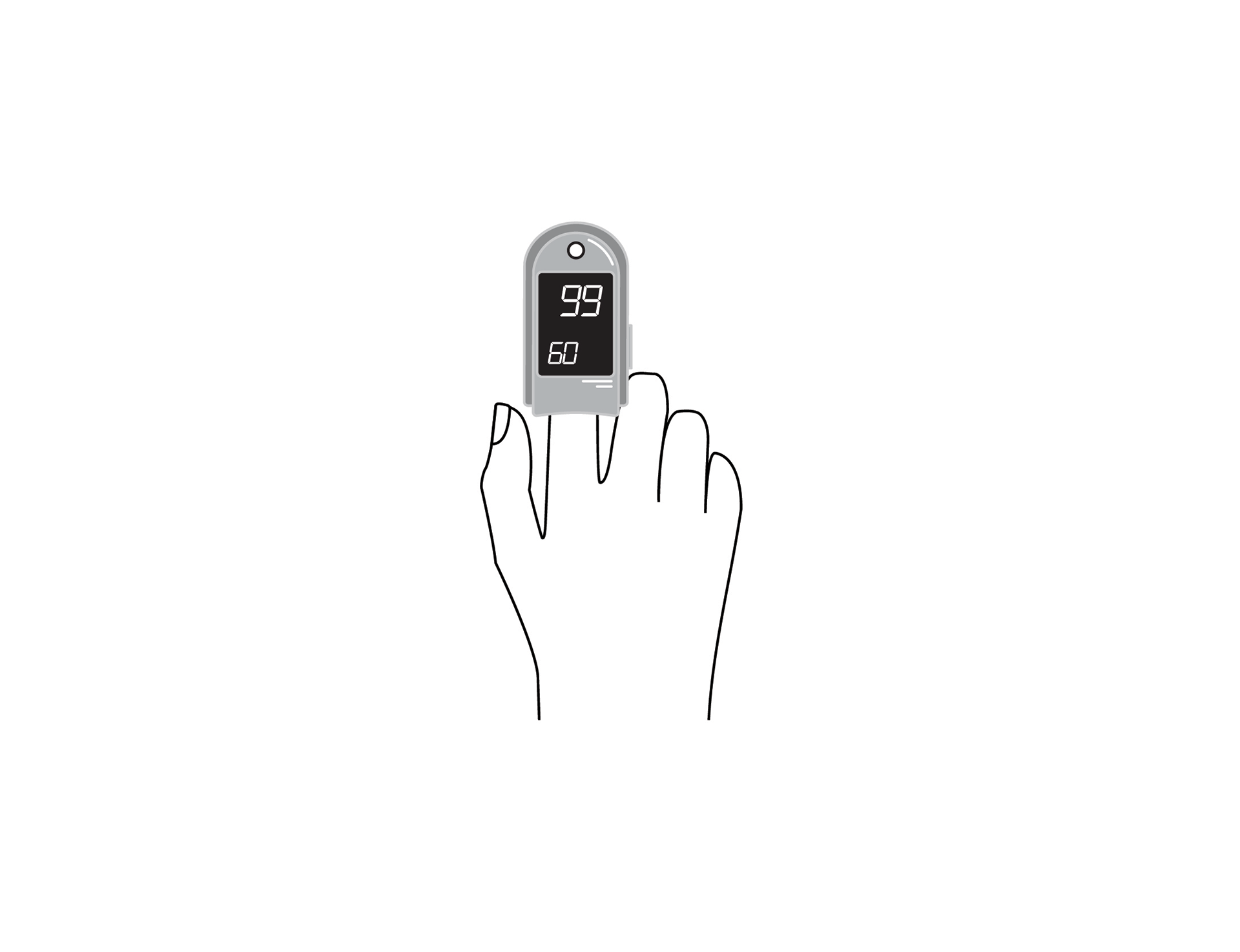
Handheld, portable, battery powered pulse oximeter, continuously displaying patient oxygen saturation (SpO2) and pulse rate in real time using an external probe on the skin.
Indicative Price 298.00 USD
GENERAL DESCRIPTION
Handheld, portable, battery-powered pulse oximeter, continuously displaying patient oxygen saturation (SpO2) and pulse rate using an external probe on the skin.
TECHNICAL SPECIFICATIONS
Measurement:
SpO2 and pulse rate monitor, with plethysmography waveform, for adults, children and neonates.
SpO2 detection includes the range: 70 - 100 %. Also capable of measuring a wider range of saturation.
SpO2 resolution: 1 %.
SpO2 accuracy (in the range 70 - 100%): ± 2% under normal conditions (± 3% in low perfusion).
Pulse Rate detection includes the range 20 - 250 bpm.
Pulse Rate resolution 1 bpm.
Pulse Rate accuracy within ± 3 bpm or ± 2% in the range 20 – 250bpm for all patients and perfusion conditions.
Display:
Data refreshing rate: 1 s.
Display with main parameters: % SpO2, pulse rate, plethysmographic waveform, signal quality, alarm messages, battery state indication.
Features:
Suitable for detection in low perfusion conditions.
Design enables use in demanding environments e.g. shock, vibration.
Audible and visual alarms for low saturation and high/low pulse rate, thresholds easily settable by user.
Variable pitch to indicate changes in oxygen saturation levels.
Audible and visual alarms for sensor error or disconnected, system errors, low battery.
Alarm override and temporary silencing function.
Capable of working with adult, paediatric and neonatal reusable probes.
Usability testing as per IEC 62366-1.
Enclosure protection IP22.
Suitable for cleaning and disinfection according to manufacturer instructions.
Device and probe weight < 400 g.
Electrical characteristics:
Operated by rechargeable battery power supply. Rechargeable batteries are replaceable.
External AC battery charger, plug style and voltage as per local supply.
Less than 5 hours for a completely discharged battery to be fully charged.
Battery can generally be charged and discharged 300 times, depending on the length and frequency of use.
Running time on battery only > 12 hours.
ENVIRONMENTAL CONDITIONS
Operating conditions: 5 to 40°C, 10 to 95% relative humidity.
Storage conditions: -20 to 70°C, 10 to 95% relative humidity.
SUPPLIED WITH
2 x each of adult and pediatric sized reusable probes, i.e. 4 probes in total; probe cable length ≥ 1m.
2 x sets of rechargeable batteries.
1 x battery charger.
1 x carry case.
1 x set user and maintenance manuals in English/French/Spanish at minimum. Other languages can be requested. If digital manuals will be provided, then a quick reference guide in English/French/Spanish languages which clearly indicates the URL to access complete manuals will be supplied with each device.
1 x certificate of calibration and inspection.
1 x list of all spares and accessories, with part numbers and contact details for parts supply.
1 x document with contact details of manufacturer, supplier and local service agent.
ITEM THAT MAY BE REQUIRED BUT IS NOT INCLUDED
S0845219 Sensor, reusable, pulse ox., neonate
WARRANTY
Includes 2-year standard warranty for the device and all accessories supplied with it, dated from the completion of the terms of delivery.
AFTERSALES AND TRAINING SERVICES
- Online training available via YouTube link.
- 1 x free of charge, face-to-face user and maintenance training for each country ordering a minimum of 500 pulse oximeters. No limit on the number of people to be trained.
Training at additional locations can be provided at cost upon confirmation from supplier.
ESTIMATED LIFE SPAN
5 years
WEIGHT & VOLUME (Packaged)
Weight: 0.99kg
Volume: 4.8dm³
ESTIMATED LEAD TIME
6 - 12 weeks
MATERIAL SAFETY DATA SHEET INFORMATION (MSDS)
Li-Ion (Lithium-Ion) battery, UN 3480 – 3481 Class 9, Packing. Group II
MARKET CLEARANCE AND DEVICE CLASSIFICATION
CE certified under the EU MDD 93/42/EEC or MDR 2017/745/EEC as Class IIb device.
QUALITY MANAGEMENT SYSTEM
Manufacturer is certified to ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
SAFETY AND PRODUCT STANDARDS
Complies with the following standards:
ISO 14971 Medical Devices -- Application of risk management to medical devices.
ISO 80601-2-61 Medical electrical equipment -- Part 2-61: Particular requirements for basic safety and essential performance of pulse oximeter equipment.
IEC 60601-1 Medical electrical equipment -- Part 1: General requirements for basic safety and essential performance.
IEC 60601-1-2 Medical electrical equipment -- Part 1-2: General requirements for basic safety and essential performance -- Collateral standard: Electromagnetic compatibility -- Requirements and tests.
Related Products