S0845219
Sensor, reusable, pulse ox., neonate
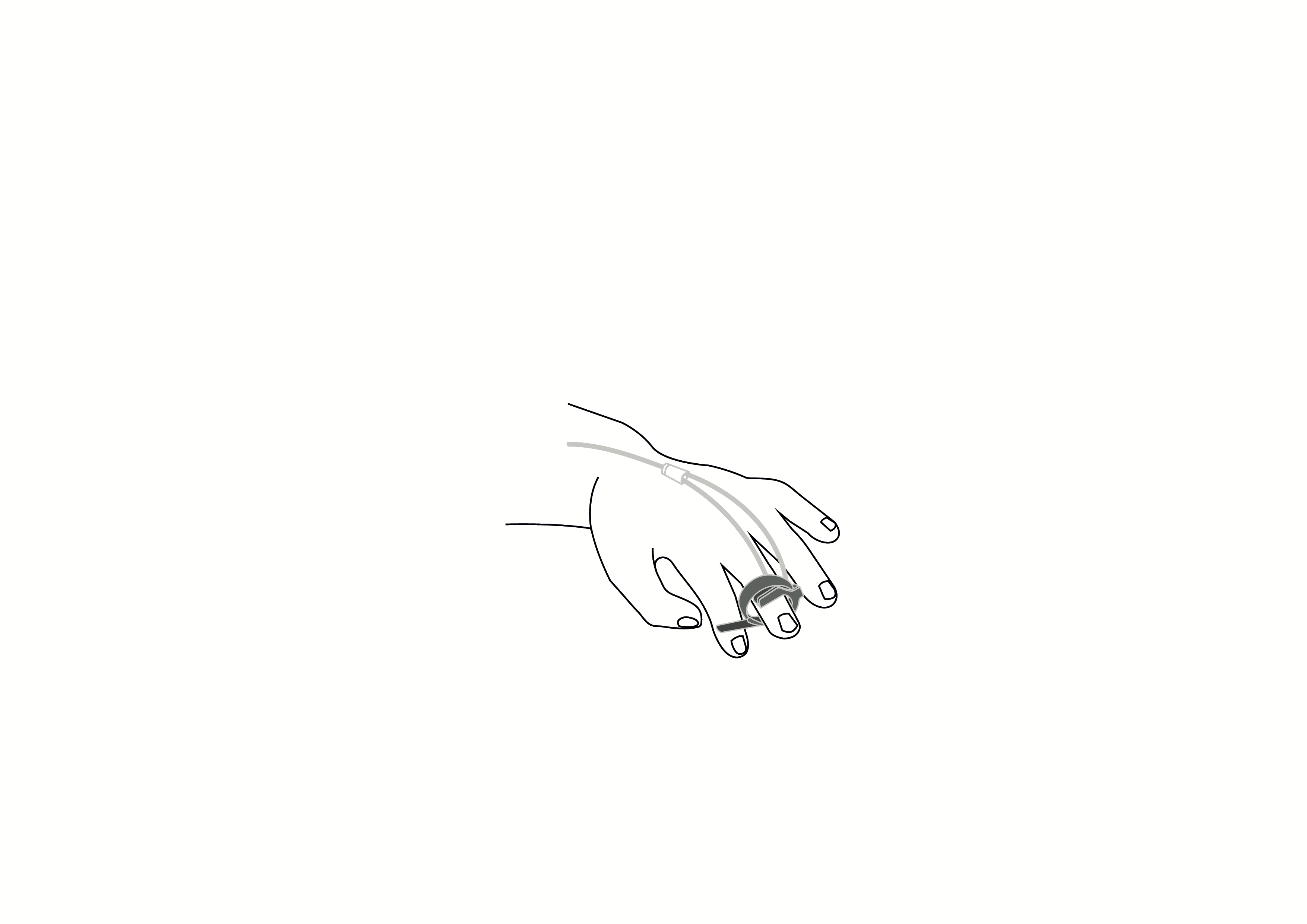
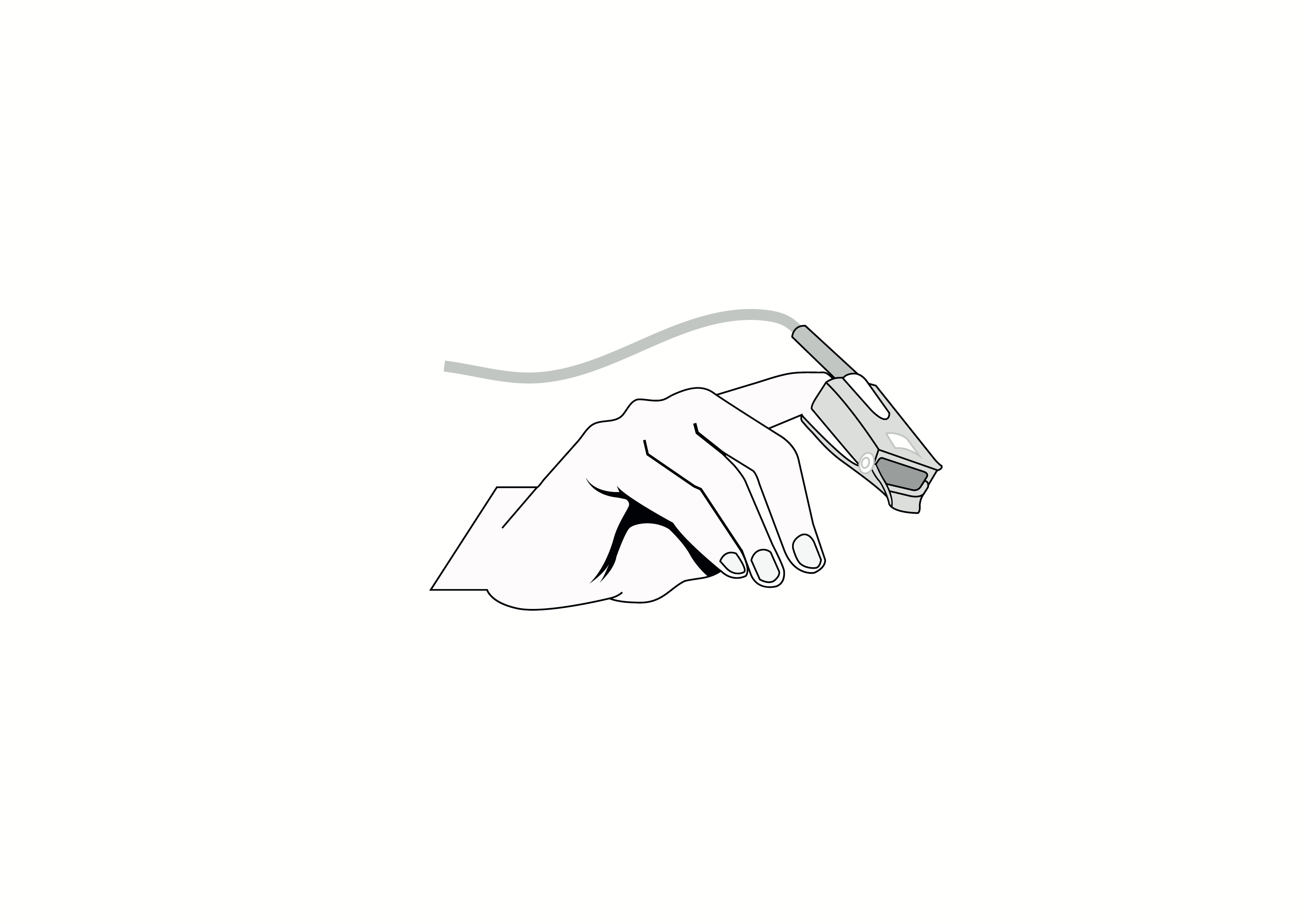
Reusable Y-style pulse oximeter sensor with wrap-around belt, Nellcor compatible, appropriate for use with neonates.
Indicative Price 26.00 USD
GENERAL DESCRIPTION
Reusable Y-style pulse oximeter sensor with wrap-around belt, Nellcor compatible, appropriate for use with neonates. Acare reference ASWNR-D3.
TECHNICAL SPECIFICATIONS
Reusable sensor indicated for spot-checking or continuous, non-invasive measurement of arterial oxygen saturation (SpO2) and pulse rate for use with neonates in operating rooms, recovery rooms, intensive care units (ICUs) and wards.
Nellcor standard comatible sensor.
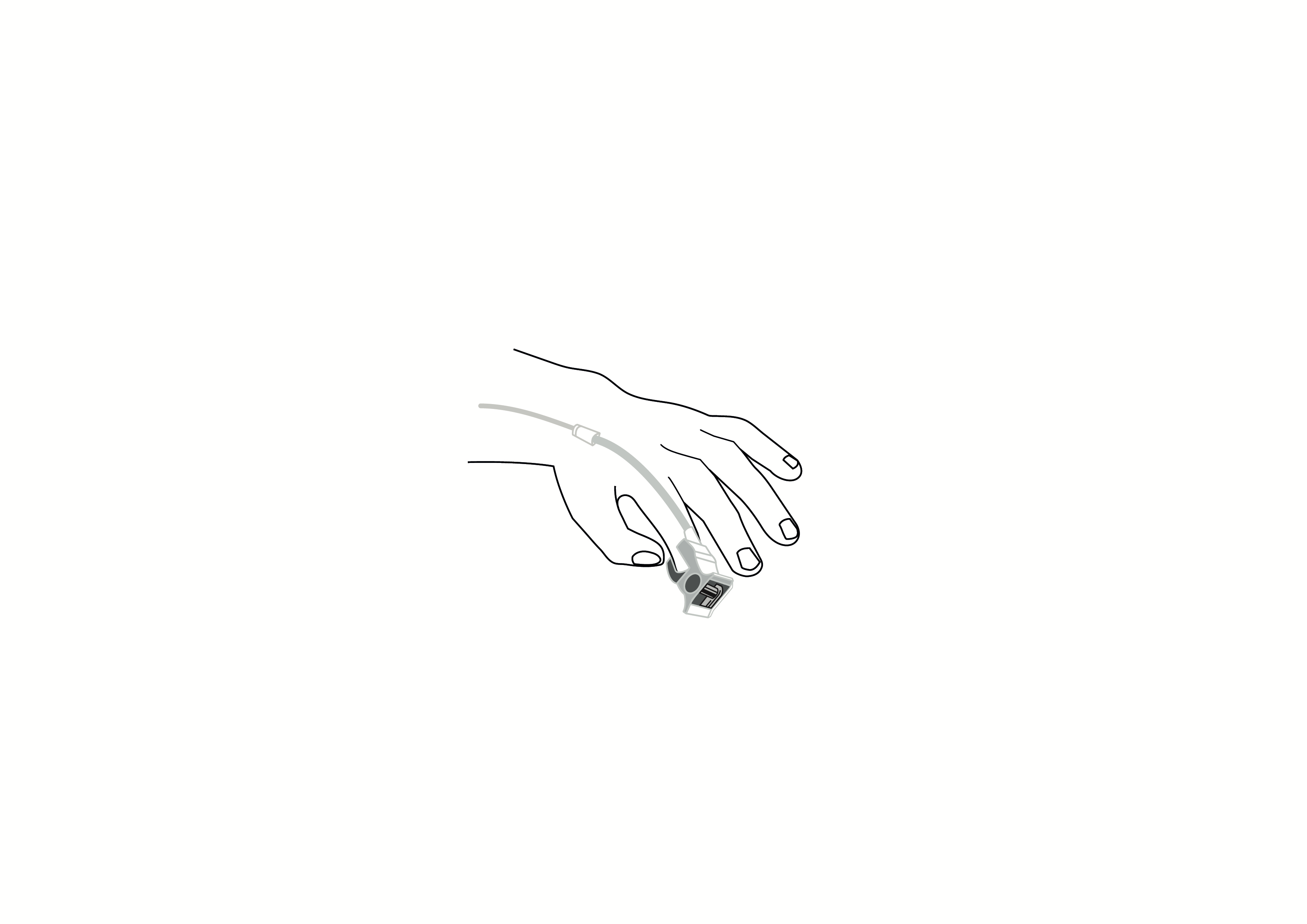
Plug type: DB9 pin
Cable length: 3.0m
Patient weight: 3 - 20kg
Design: Reusable, Y-style with built-in wrap-around belt for use on big toe or thumb
Prior to using this sensor, the user should read and understand the Instruction Manual for the pulse oximeter and the Directions for Use included with the reusable sensor.
ENVIRONMENTAL CONDITIONS
Storage Temperature: -40°C to +50°C
Storage Humidity: ≤ 93%
SUPPLIED WITH
1 x Instructions for use in English/French/Spanish at minimum. Other languages can be requested. If digital manuals will be provided, then a quick reference guide in English/French/Spanish languages which clearly indicates the URL to access complete manuals will be supplied with each device.
WARRANTY
Includes 12-month standard warranty, dated from the completion of the terms of delivery.
SHELF LIFE
3 years
WEIGHT & VOLUME (Packaged)
Weight: 0.073kg
Volume: 0.34dm³
ESTIMATED LEAD TIME
6 - 12 weeks
MARKET CLEARANCE AND DEVICE CLASSIFICATION
CE certified under the EU MDD 93/42/EEC or MDR 2017/745/EEC as Class IIb device.
QUALITY MANAGEMENT SYSTEM
Manufacturer is certified to ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
SAFETY & PRODUCT STANDARDS
Complies with the following standards:
ISO 14971 Medical Devices -- Application of risk management to medical devices.
ISO 80601-2-61 Medical electrical equipment -- Part 2-61: Particular requirements for basic safety and essential performance of pulse oximeter equipment.
IEC 60601-1 Medical electrical equipment -- Part 1: General requirements for basic safety and essential performance.
IEC 60601-1-2 Medical electrical equipment -- Part 1-2: General requirements for basic safety and essential performance -- Collateral standard: Electromagnetic compatibility -- Requirements and tests.
Related Products