S0845218
Sensor, reusable, pulse ox., pediatric
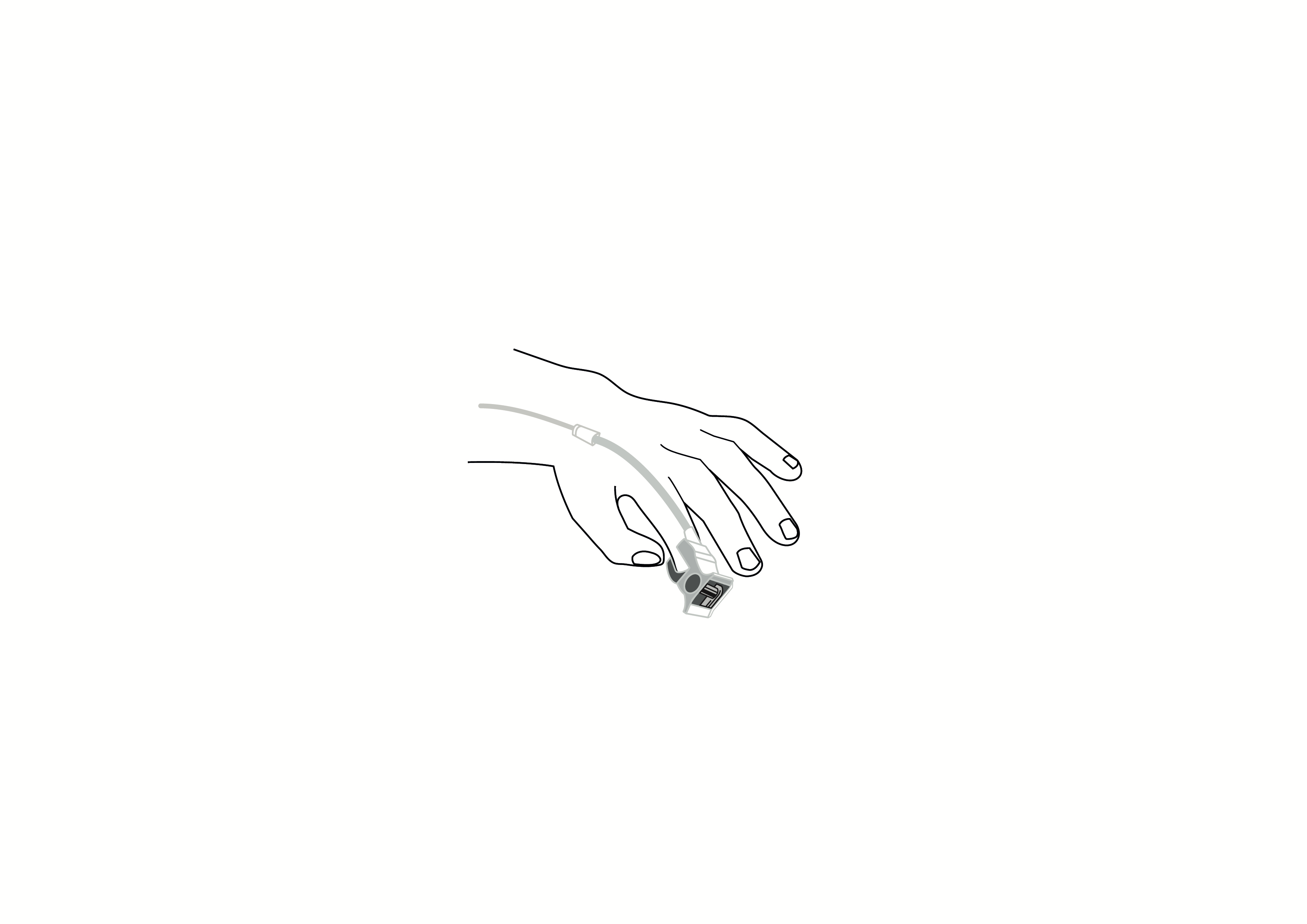
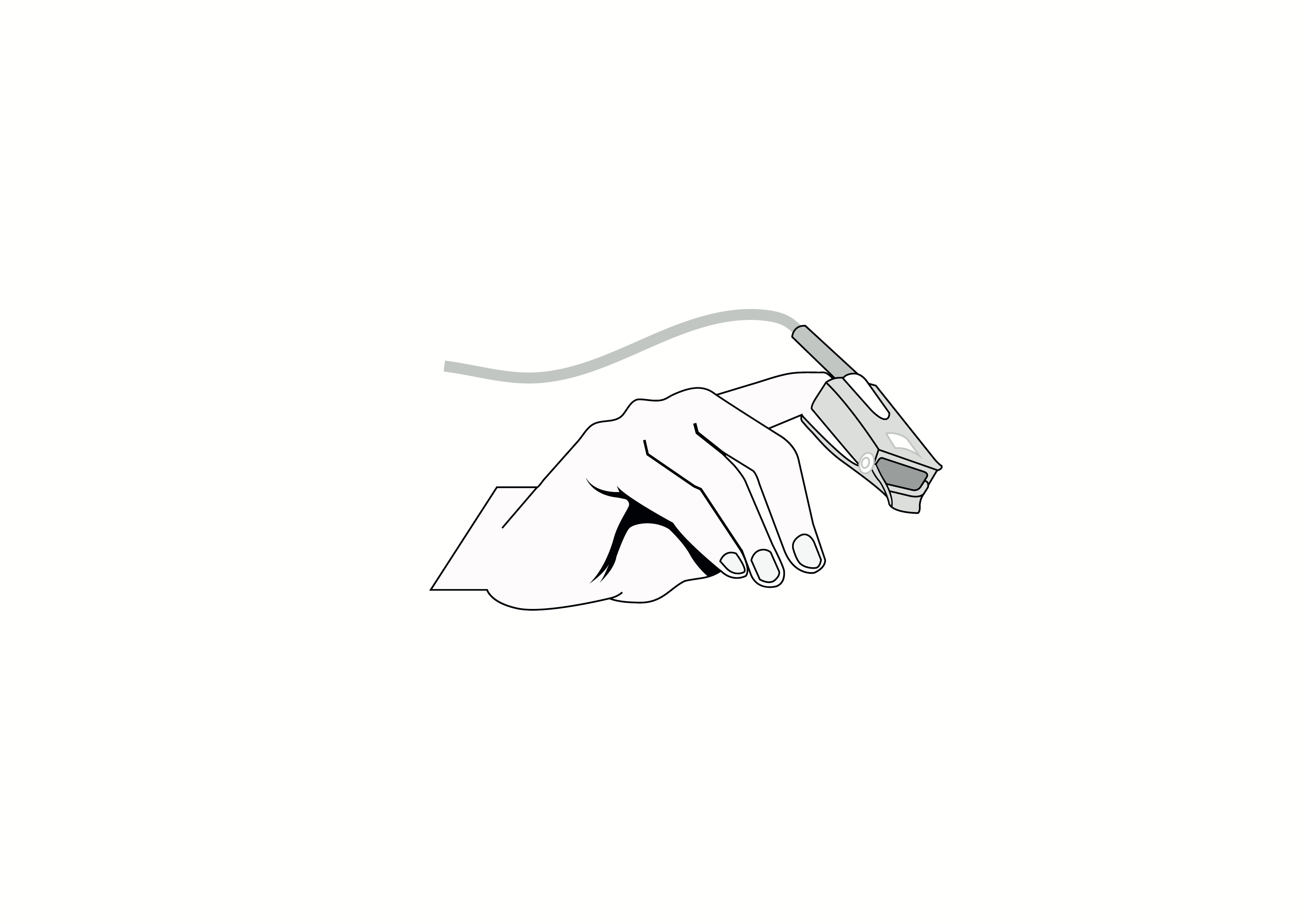
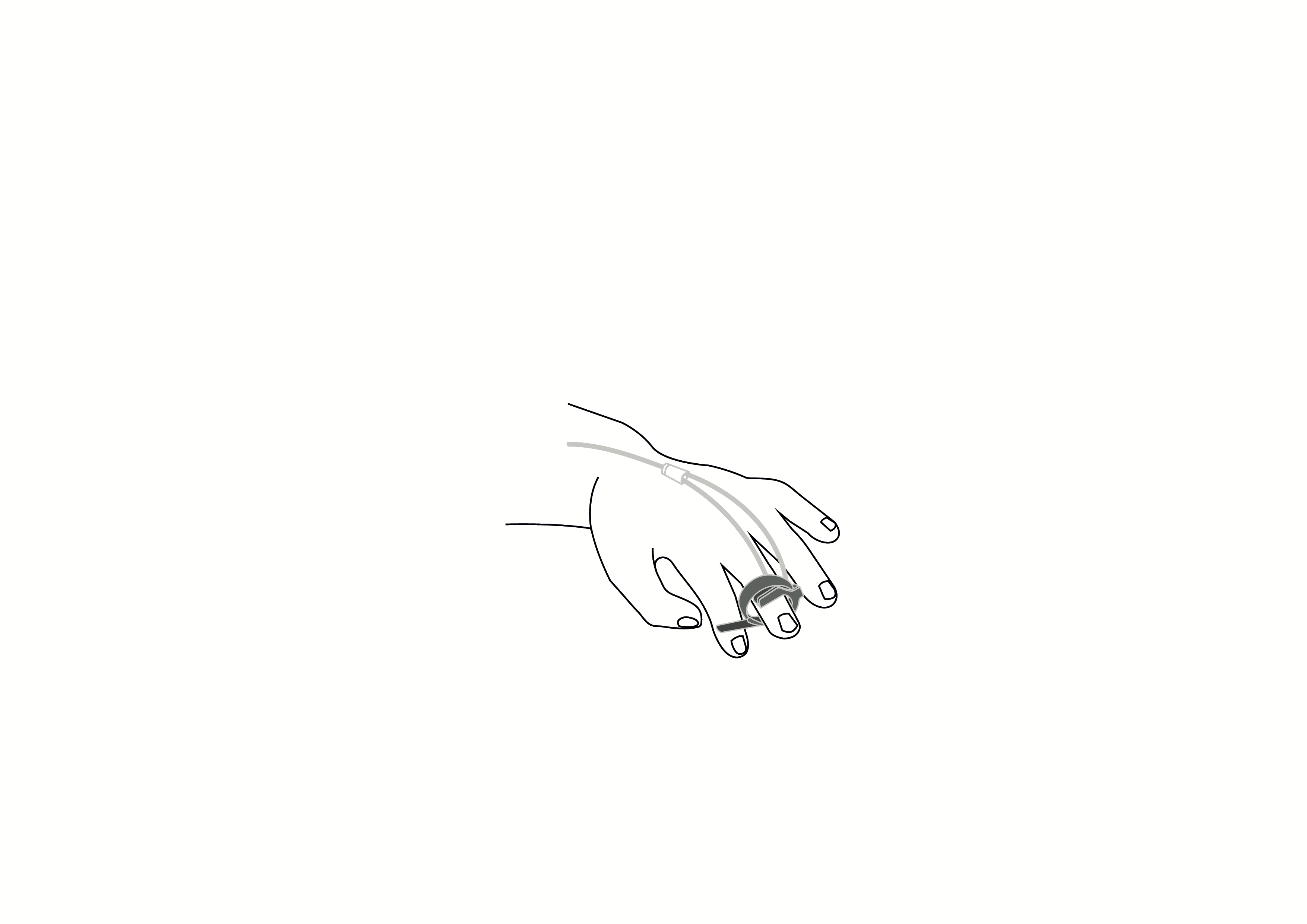
Reusable finger clip pulse oximeter sensor, Nellcor compatible, appropriate for use with children.
Indicative Price 25.00 USD
GENERAL DESCRIPTION
Reusable finger clip pulse oximeter sensor, Nellcor compatible, appropriate for use with children. Acare reference ASPNR-D3.
TECHNICAL SPECIFICATIONS
Reusable sensor indicated for spot-checking or continuous, non-invasive measurement of arterial oxygen saturation (SpO2) and pulse rate for use with children in operating rooms, recovery rooms, intensive care units (ICUs) and wards.
Nellcor standard compatible sensor.
Plug type: DB9 pin
Cable length: 3.0m
Patient weight: 10 - 40kg
Design: Reusable, finger clip
Prior to using this sensor, the user should read and understand the Instruction Manual for the pulse oximeter and the Directions for Use included with the reusable sensor.
ENVIRONMENTAL CONDITIONS
Storage Temperature: -40°C to +50°C
Storage Humidity: ≤ 93%
SUPPLIED WITH
1 x Instructions for use in English/French/Spanish at minimum. Other languages can be requested. If digital manuals will be provided, then a quick reference guide in English/French/Spanish languages which clearly indicates the URL to access complete manuals will be supplied with each device.
WARRANTY
Includes 12-month standard warranty, dated from the completion of the terms of delivery.
SHELF LIFE
3 years
WEIGHT & VOLUME (Packaged)
Weight: 0.081kg
Volume: 0.34dm³
ESTIMATED LEAD TIME
6 - 12 weeks
MARKET CLEARANCE AND DEVICE CLASSIFICATION
CE certified under the EU MDD 93/42/EEC or MDR 2017/745/EEC as Class IIb device.
QUALITY MANAGEMENT SYSTEM
Manufacturer is certified to ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
SAFETY AND PRODUCT STANDARDS
Complies with the following standards:
ISO 14971 Medical Devices -- Application of risk management to medical devices.
ISO 80601-2-61 Medical electrical equipment -- Part 2-61: Particular requirements for basic safety and essential performance of pulse oximeter equipment.
IEC 60601-1 Medical electrical equipment -- Part 1: General requirements for basic safety and essential performance.
IEC 60601-1-2 Medical electrical equipment -- Part 1-2: General requirements for basic safety and essential performance -- Collateral standard: Electromagnetic compatibility -- Requirements and tests.
Related Products