S0002048
CPAP,bubble,medical,new-born
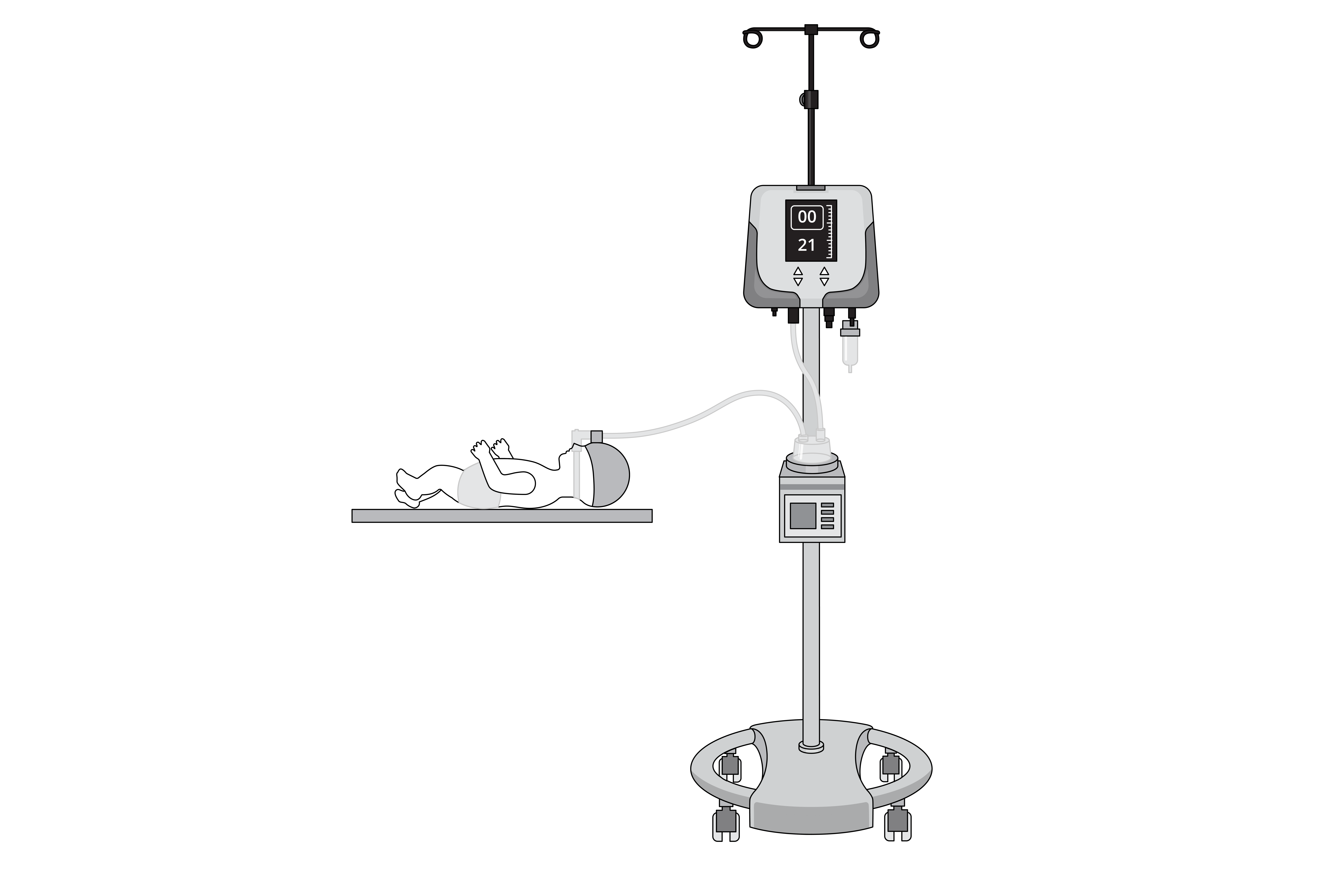
Bubble CPAP non-invasive respiratory support system, for all normal , premature and low birth-weight neonates. Comes with humidifier , air/oxygen mixer, and accessories.
Indicative Price 5,117.00 USD
GENERAL DESCRIPTION
Continuous Positive Airway Pressure (Bubble CPAP) system for the non-invasive respiratory support of normal, premature or low-birth weight neonate with spontaneous breathing.
IMPORTANT
Training and installation are not included with the device and must be requested separately from the supplier or manufacturer. Improper operation or maintenance can result in harm to patients and/or operators, including death. Therefore, UNICEF strongly recommends the inclusion of installation and training services for both operators and technicians, unless identical models of these devices are already in use at the intended installation site. Neither UNICEF nor the manufacturer can assume responsibility for any consequences arising from incorrect operation and/or maintenance. Training and installation will have to be quoted separately; the number of operators and technicians to be trained, and the locations where these trainings will have to take place, are required in order to obtain a quotation.
INTENDED USE
An electrically powered device designed to deliver high-flow (exceeding peak inspiratory flow) heated and humidified ambient air or air/oxygen to a neonatal patient as part of non-invasive ventilation (NIV); it may additionally be used with a water tank to produce bubble continuous positive airway pressure (bubble CPAP). It consists of a medical air input, an oxygen input, an electronic gas mixer, a heating element, and humidification chamber. It is intended for use by a healthcare provider on a spontaneously breathing patient in hospital settings.
TECHNICAL SPECIFICATIONS
The unit supports bubble CPAP Mode.
Specifically for support of neonates and newborns.
The pressure/flow of the CPAP generator is regulated electronically.
The unit has an integrated humidifier.
The unit is equipped with an electronic air/oxygen mixer.
The unit has an integrated FiO₂ analyser.
The unit accepts inlet gas supply pressures between 3.5 – 6 bar (43 to 87 psi).
O2 and air connections compliant with German Standard, ISO 9170-1, DIN 13620-2 and CE listed.
Supplied with pole mounting system, wheeled and with brakes.
The pole mounting system is equipped with 4 antistatic swivel castors, of which two castors. have been equipped with brakes.
Equipped with an air filter and a water trap.
The unit accepts other than the manufacturer patient circuits.
Suitable for heated and non-heated closed patient circuits
All components of the system (single-use filters and patient circuits excluded) are suitable for disinfection with hospital grade products.
Minimum CPAP pressure range is adjustable between 0 and 10 cm H2O.
Maintains constant CPAP at outlet.
A minimum output flow range adjustable between 0 – 15 L/min.
Oxygen concentration adjustable between 21 - 100 %.
Pressure indicator in cmH2O.
Noise emission less than 50 dB.
Including a clamp for a rail and/or pole.
External electric heating and humidifying chamber.
Relative humidity output up to 100%.
Adjustable output temperature up to 36°C.
Power requirements: 100 - 240 Volts - 50/60 Hz (not necessarily in a single unit).
ALARMS
Alarms are audible as well as visual.
The unit has alarms for the following:
- High temperature.
- High humidity.
- System failure.
- High/low oxygen concentration (FiO₂).
- High airway pressure.
- Failure in either air or oxygen supply.
- Mains power failure.
- Low battery.
SAFETY PROVISIONS
The unit has a built-in safety feature to prevent overpressure in the patient circuit.
Overheating protection.
The unit is equipped with a rechargeable battery which provides for a minimum of 1-hour autonomous operation.
In case of power failure, the unit switches automatically to battery power.
The unit automatic recharges on reconnection to mains.
SUPPLIED WITH
Instructions for assembly, use and maintenance in English.
1 x Plastic protective dustcover.
4 x reusable patient circuits.
2 sets of extra small size reusable CPAP head bonnets.
2 sets of small size reusable CPAP head bonnets.
2 sets of medium size reusable CPAP head bonnets.
10 x nasal cannulas, soft and atraumatic, extra small size for premature neonates.
10 x nasal cannulas, soft and atraumatic, small size for term and older infants.
1 x set of hoses for connecting external oxygen and medical air supply, length > 2 m.
OPTIONAL ACCESSORIES AVAILABLE
At extra costs additional optional features can be requested:
- A electrical medical air compressor with the following minimal features: Delivers medical grade air with sufficient flow to support the CPAP. Replacement of internal filters is possible without removing the compressor. Equipped with a washable air filter.
ESTIMATED LIFE SPAN
8 years.
WARRANTY
Two years from shipping date.
ENVIRONMENTAL CONDITIONS
- Operating conditions: 5°C - 40°C / 0% – 95% RH.
- Storage conditions: 5°C - 45°C / 10% – 95% RH.
- Atmospheric pressure: 700 ~ 1060hPa.
- Ingress protection rating: IPX0.
WEIGHT AND VOLUME (packaged).
Weight: 32.00 kg.
Volume: 338.688 dm³.
ESTIMATED DELIVERY LEAD TIME
120 days.
INSTALLATION REQUIREMENTS
Assembly and commissioning this product should be carried out by qualified technician.
TRAINING REQUIREMENTS
User training prior to utilization is recommended.
MAINTENANCE/USER REQUIREMENTS
As per user and service manuals.
RELATED PRODUCTS:
S0002031 Monitor,patient,portable,w/access.
S0002033 Pulse oximeter,portable,w/access.
ALTERNATIVE PRODUCTS
S0004002, CPAP,newborn,w/compressor & O₂ concentr.
S0004057, CPAP,bubble,newborn,wcompressor & SpO2
COMPONENT OF A KIT
No part of a kit.
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier (if not the manufacturer) at a minimum is certified for ISO 9001 Quality management systems – Requirements.
CLASSIFICATION
Classified either under EU MDD 93/42/ECC, or under EU MDR 2017/745 as Class IIb device.
SAFETY & PRODUCT STANDARDS
- IEC 60601-1:2005 + A1:2012(E) Medical electrical equipment - Part 1: General requirements for basic safety and essential performance.
- IEC 60601-1-2:2014 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests.
- IEC 60601-1-6:2010 Medical electrical equipment - Part 1-6: General requirements for basic safety and essential performance
Related Products