S0004020
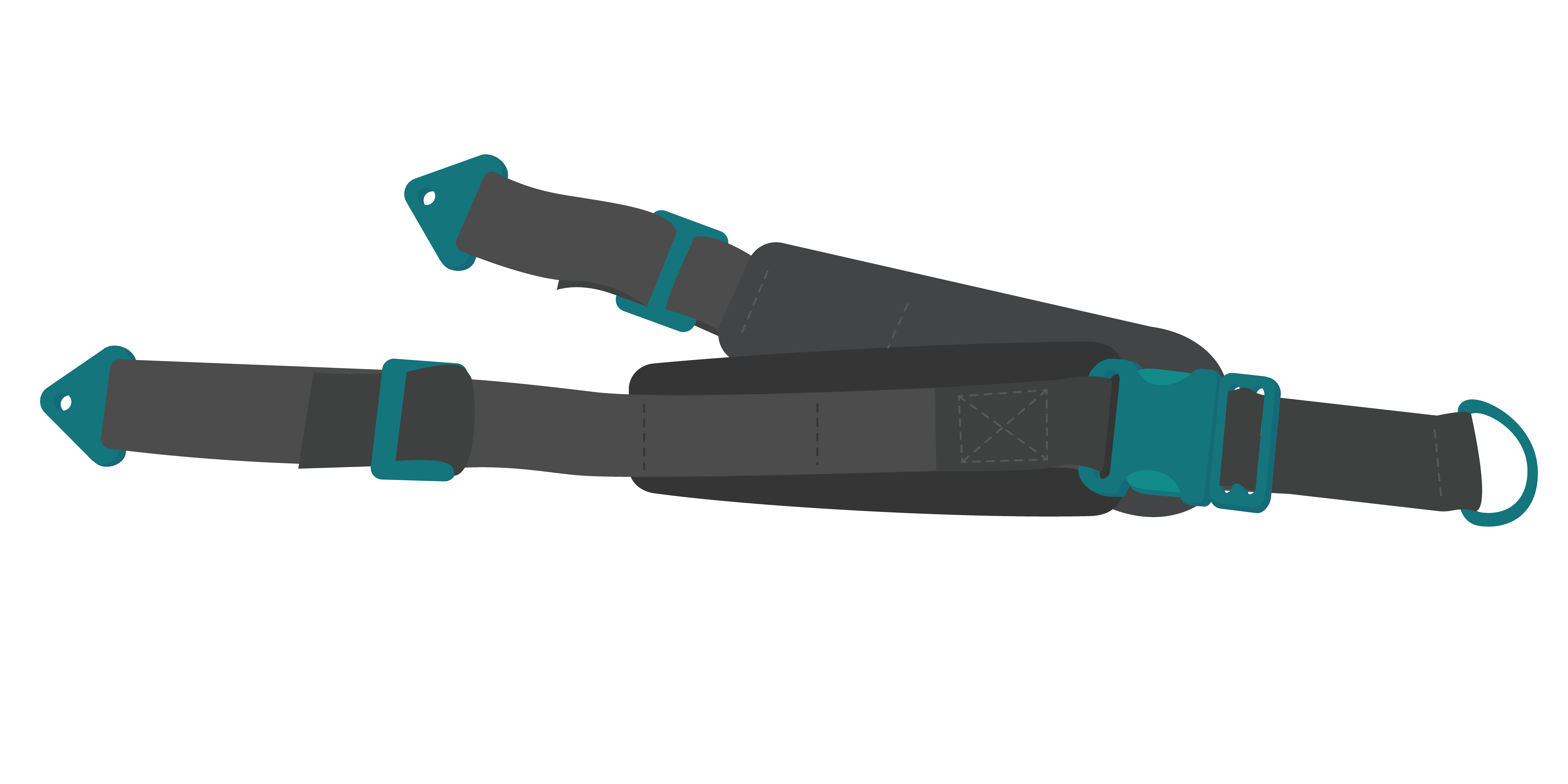
Strap pelvis/hip, wheelchair
Pelvis strap:
FUNCTION AND POSTURE SUPPORT: For CHILD and ADULT wheelchair users to optimise posture support of the pelvis. Prevents the pelvis from sliding forward or rotating back (posterior tilt). Durable, adjustable straps with universal design to be fitted to a range of wheelchairs.
ENVIRONMENT: For indoor and outdoor use.
Indicative Price 34.00 USD
GENERAL DESCRIPTION
Strap, pelvis or hip, for wheelchair
IMPORTANT INFORMATION
Minimum order quantities may apply depending on model selected.
Inappropriate prescription and fitting of wheelchairs, cushions and accessories may harm the user’s health and/or function. Therefore, this product should only be purchased after consultation with the Medical Devices Unit in Supply Division.
INTENDED USE
Pelvis strap:
FUNCTION AND POSTURE SUPPORT: For CHILD and ADULT wheelchair users to optimise posture support of the pelvis. Prevents the pelvis from sliding forward or rotating back (posterior tilt). Durable, adjustable straps with universal design to be fitted to a range of wheelchairs.
ENVIRONMENT: For indoor and outdoor use.
TECHNICAL SPECIFICATIONS
One unit with two separate sections
Length adjustable to optimise fit after fastened
Padded over thigh area
Fastener: user-friendly, quick-release, buckle type system
Skin protection through:
- Padding which contours to the body
- Design to prevent curling of edges, bunching, wrinkling, deforming or fraying and/or localised pressure points
Washable
Eyelets in strap for mounting OR mounting mechanism for wheelchair frame, depending on model selected
Mounting length adjustable
Size and dimensions:
3-4 sizes to fit wheelchair with seat width ranging between 250-500 mm depending on model selected
Strap: 25-40 mm OR 25-50 mm wide depending on model and size selected
Padding: 65-80 mm OR 55-75 mm wide depending on model and size selected
ASSEMBLY
Fully assembled
SUPPLIED WITH
Standard components to attach to wheelchair depending on model selected
Detailed illustrated instructions to mount/attach to wheelchair
Detailed illustrated instructions for safe use
WARRANTY
Limited warranty to cover defects in material and workmanship only. Warranty excludes normal wear or damage following inappropriate use or lack of maintenance.
Warranty: 1-2 years depending on model selected
MINIMUM ORDER QUANTITIES
Minimum order quantities may apply depending on model selected
PACKAGING, LABELLING, INSTRUCTIONS:
Please note: Minimum order quantities may apply depending on model selected
One (1) unit per box
Dimensions:
- Unit Weight in Kg (including its packaging): 0.5 kg
- Unit Volume in M3 (including its packaging) 0.01 m³
Labelling: Compliance with EAN 128 bar code requirements
ESTIMATED LEAD TIME
60 days
ENVIRONMENTAL CONDITIONS
Operating conditions: 0 to 50 degrees Celsius; 20 to 60% RH
Storage conditions: 0 to 50 degrees Celsius; 10 to 85% RH
ESTIMATED LIFE SPAN
1-5 years depending on model selected
USER FITTING AND TRAINING REQUIREMENTS
Users to be fitted and trained by trained wheelchair service personnel.
MAINTENANCE REQUIREMENTS
Product maintenance as per manufacturer’s instructions
RELATED PRODUCTS
S0004004 Wheelchair, assistnt controlld,transport
S0004005 Wheelchair,active urban,fold,more support
S0004006 Wheelchair, active urban, folding
S0004007 Wheelchair, active urban, rigid
S0004008 Wheelchair, active dual terrain, folding
S0004009 Wheelchair, active dual terrain, rigid
S0004010 Wheelchair, active rough terrain
S0004011 Wheelchair, postural support
S0004012 Cushion,wheelchair, posture, layered
S0004014 Cushion,wheelchair,pressure, mod layered
S0004015 Cushion,wheelchair,pressure,mod no layer
S0004018 Cushion,wheelchair,pressure,deep,section
ALTERNATIVE PRODUCTS
S0004021, Strap lower leg/calf, wheelchair
S0004022 Strap ankle/foot, wheelchair
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier is certified for ISO 9001 Quality management systems – Requirements.
MARKET CLEARANCE AND DEVICE CLASSIFICATION
CE certified under the EU MDR 2017/745 as Class I device.
SAFETY AND PRODUCT STANDARDS
ISO 16840-3:2014
NOMENCLATURE
GMDN Code:
13356
Related Products