S0002046
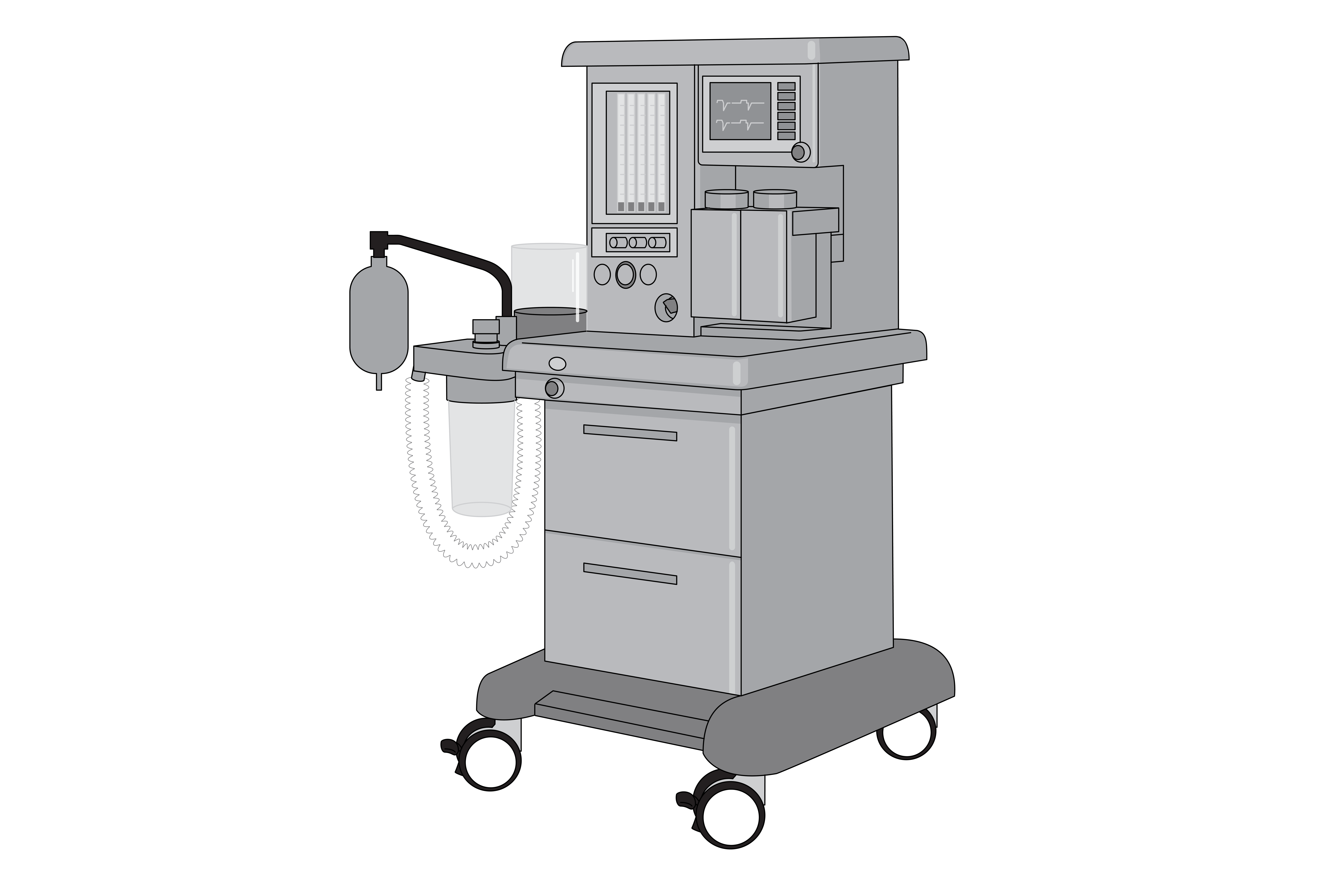
Anaesthesia machine,closed circuit,w/acc
Anaesthesia unit, closed or semi-closed circuit, AC and battery powered , inlcuding vaporizer, mixer and other accessories.
Indicative Price 11,729.00 USD
GENERAL DESCRIPTION
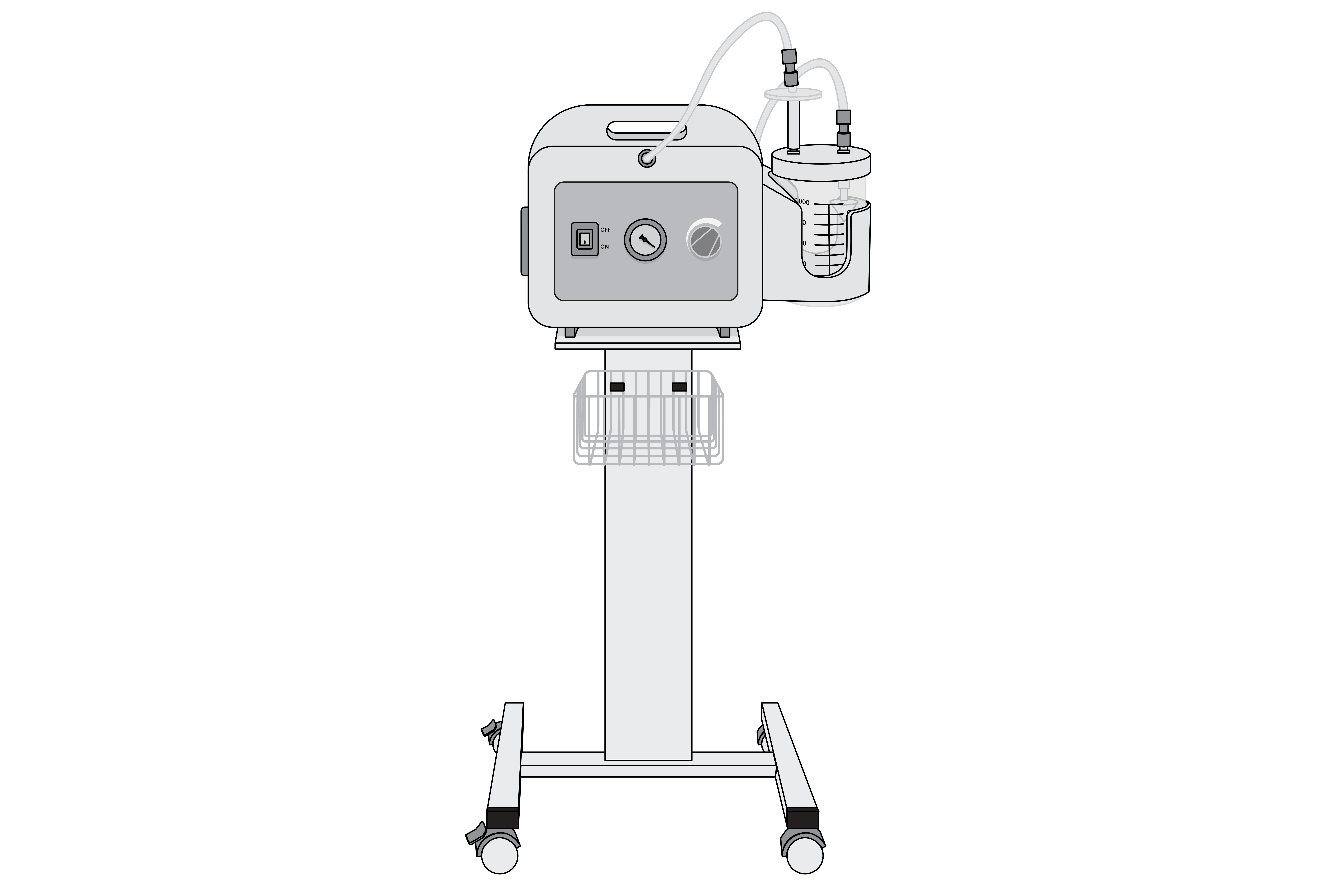
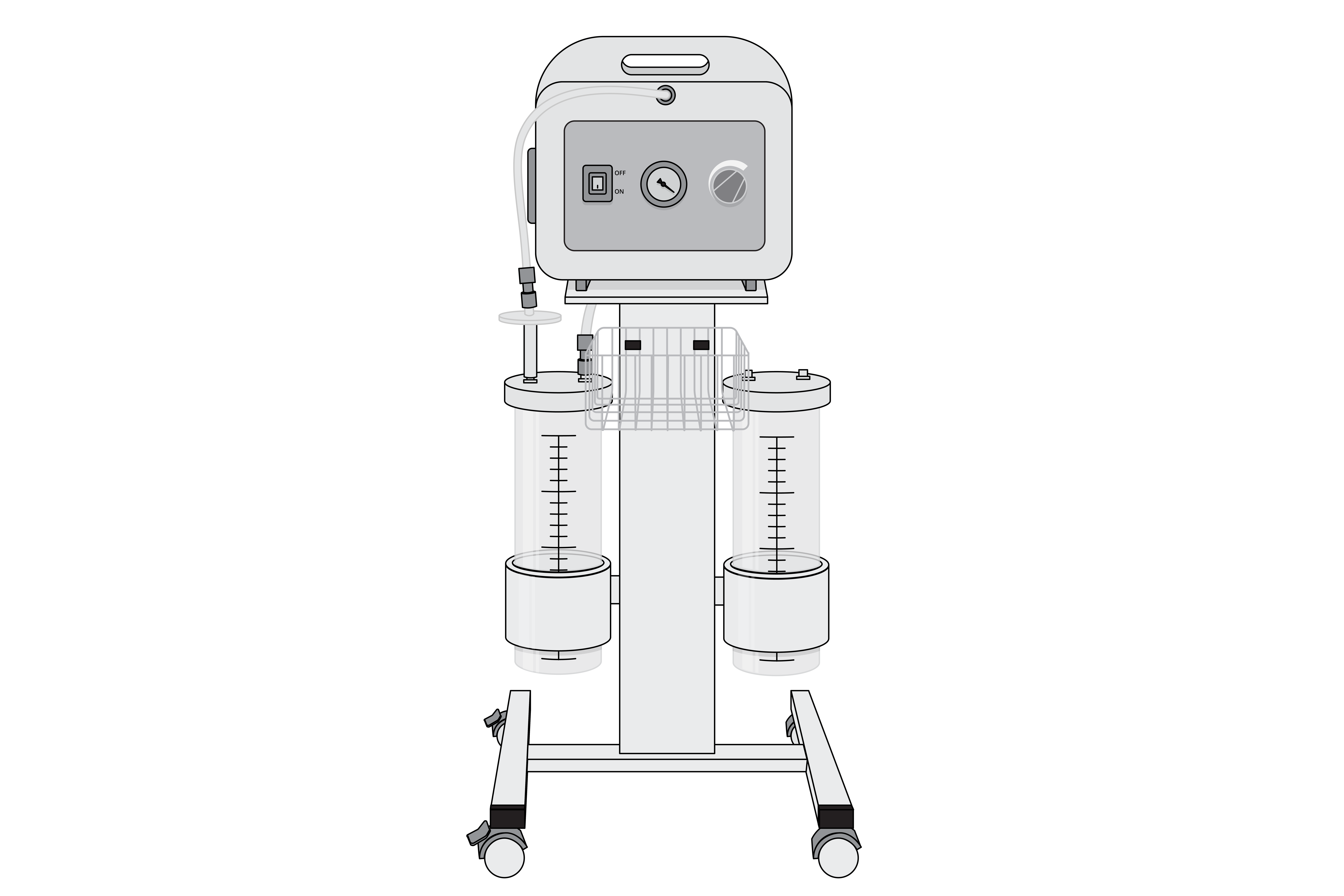
Anaesthesia machine closed or semi-closed circuit configuration.
IMPORTANT
Training and installation are not included with the device and must be requested separately from the supplier or manufacturer. Although these devices are designed to be life-sustaining, improper operation or maintenance can result in harm to patients and/or operators, including death. Therefore, UNICEF strongly recommends the inclusion of installation and training services for both operators and technicians, unless identical models of these devices are already in use at the intended installation site. Neither UNICEF nor the manufacturer can assume responsibility for any consequences arising from incorrect operation and/or maintenance Training and installation will have to be quoted separately and will require the number of operators and technicians to be trained and the locations where these trainings will have to take place.
NOTE:
- Oxygen concentrators cannot be used as an source for oxygen for this ventilator.
- No patient interfaces included with the device, these should be ordered separately.
For any inquiries regarding this device, please contact the UNICEF Supply Division via email at: medical-technical@unicef.org
INTENDED USE
Anaesthesia units dispense a mixture of gases and vapours and vary the proportions to control a patient's level of consciousness and/or analgesia during surgical procedures. Anaesthesia units primarily perform the following four functions:
- Provide oxygen (O₂) to the patient
- Blend gas mixtures, in addition to O₂, that include air or nitrous oxide (N₂O) along with an anaesthetic vapor.
- Facilitate spontaneous, controlled, or assisted ventilation while using these gas mixtures.
- Reduce, if not eliminate, anaesthesia-related risks to the patient and clinical staff.
The patient is anesthetized by inspiring a mixture of O₂, the vapor of a volatile liquid halogenated hydrocarbon anaesthetic, and, if necessary, N₂O and other gases. Because normal breathing is routinely depressed by anaesthetic agents and by muscle relaxants administered in conjunction with them, respiratory assistance is also provided via either an automatic ventilator or by manual compression of the reservoir bag.
TECHNICAL SPECIFICATIONS
Suitable for adult, paediatric and newborn patients.
The unit is mounted on a trolley with minimum of four (4) anti-static swivel castors.
A minimum of two of the castors are provided with breaks.
The unit is equipped with an upper shelf.
The cart has been proved with handles for manoeuvring.
A side rail for mounting accessories is included.
The unit has an adjustable patient-circuit support arm.
The unit is provided with a minimum of three (3) gas inlets (O₂, N₂O and Air).
Gas inlet connections are compliant with DISS or NIST (needs to be specified when ordering).
The unit is provided with a minimum of two (2) gas cylinder yokes (O₂, and N₂O).
Holders for bottles are to include a secure locking mechanism.
The minimum gas supply input pressure range should be between 2.8 – 6 bar (41 - 87 psi).
The unit are equipped with gas supply gauges with scales allowing easy readout.
All pipeline connections have diameter-indexed safety systems (DISSs), or another means of preventing connection of dangerous gases.
The unit is equipped with a minimum of four (4) analogue rotameters, two (2) for O₂, one (1) for air and one (1) for N₂O with programmed parameters visualization.
The O₂ and N₂O flowmeters have a minimum range of 0.0 – 10 L/min, and a resolution of at least 0.2 L/min.
Externally supplied N₂O, O₂, and air mixture ratios are fully controllable. There are separate controls for the flow of: O₂, N₂0, and anaesthetic agents.
Flows and the mixture ratios determined from flowmeter settings are accurate to within ± 10% of set values or better.
The unit has the ability to carry out self-diagnosis and integrity testing, including a compliance and leakage test.
The unit is equipped with a minimum of two (2) vaporizer slots with a Selectatec mounting system.
The vaporizers are provided with an interlock system, preventing the use of more than one vaporizer simultaneously.
Two (2) vaporizers are standard included: one sevoflurane and one isoflurane.
The unit basic is equipped with a passive scavenging system and optional allow for an active scavenging system.
The unit is equipped with a soda lime CO₂ absorber.
The unit is equipped with a non-return and a three-way valve, including the connection tube.
A built-in rechargeable battery allows for an autonomy of at least one (1) hour.
The unit will switch back and forth between battery and mains operation in case of power failures. Recharging resumes automatically when extern power becomes available.
The unit is designed and constructed in such a way that it is able to withstand frequent disinfection with hospital-grade products.
Fuses are fitted on both live and neutral supply lines.
Power requirements: 100 - 240 Volts - 50/60 Hz (not necessarily in a single unit).
VENTILATOR
The unit should be equipped with a ventilator suitable for adult, paediatric, and newborn patients and minimally supporting the following ventilation modes:
- Volume Controlled Mode.
- Stand-by Mode.
- Manual Mode.
The unit should be able to provide a minimum guaranteed tidal volume of 20 – 1,500 mL.
The unit should be able to support a minimum ventilation frequency range between 1 – 100 bpm.
The unit supports an I/E ratio 4:1 to 1:10.
The unit supports an inspiration pressure of 5 – 70 cmH₂O.
The unit supports a peak inspiratory flow range of 1 – 70 L/min.
The unit supports PEEP with a range of 3 – 30 cmH₂O.
The unit supports pressure triggers in the range of 0 – 20 cmH₂O.
Pressure gauges have a range of 0 – 100 cmH₂O and an accuracy of: ±2.5 % or better.
MONITORED PARAMETERS
The ventilation monitors following parameters, through a combination of numerical and waveforms:
- Respiratory rate (spontaneous and mechanical).
- Tidal volume (inspired and expired).
- Minute volume (spontaneous and mechanical).
- Airway pressure.
- PEEP.
- Compliance.
- O₂ Concentration.
- I:E ratio.
- Inspiration and expiration times.
- End-tidal CO₂ (capnography).
- FiO₂ dynamic.
- Plateau pressure.
- Trend display facility for at least the last 24 hours, with minimum 5 minutes resolution.
- Automatic compliance and leakage compensation for circuit and tubes.
ALARM AND SAFETY FUNCTIONALITIES
Alarms are categorized in three categories: caution, advisory and alarm.
The unit is equipped with audio and visual alarms for the following parameters:
- High airway pressure alarm.
- Sub atmospheric pressure alarm.
- Respiratory rate alarm.
- Minute volume alarm.
- Tidal volume alarm.
- Expiratory flow alarm.
- FiO₂ supply failure alarm.
- O₂ supply failure alarm.
- Low pressure/apnoea alarm.
- External O₂ gas supply failure alarm.
- Low battery alarm.
- Power failure alarm.
- Sensor disconnected alarm.
- System or sensor failures alarm.
The unit has a provision that prevents an anaesthesia machine from being set to dispense a hypoxic mixture. The N₂O and O₂ flow controls are interlocked so that the proportion of O₂ to N₂O can never fall below a minimum value of 25% to produce a hypoxic breathing mixture.
The unit has a provision that protects the patient from inadequate O₂ supply. If the O₂ supply pressure drops below 1.7 - 2.1 Bar (25 to 30 psi), the unit decreases or shuts off the flow of other gases and activates an alarm.
The unit is equipped with an Adjustable Pressure Limiting (APL) valve to prevent delivery from too high-pressured gas.
The unit should have a provision for emergency O₂ by-pass.
DISPLAYED PARAMETERS
The unit is equipped with a flat panel colour display indicating the following parameters and traces:
Three (3) traces against time: pressure, volume, and flow.
Two (2) two-axis displays: Pressure-Volume and Flow-Volume.
Values for all monitored parameters.
Alarm settings for all monitored parameters.
Alarms or errors in ventilation or anaesthesia parameters.
Current time.
Ventilator mode.
Battery status.
System events.
SUPPLIED WITH
Instructions for assembly, use and maintenance in English.
1 x Plastic protective dustcover.
1 x newborn reusable breathing circuit (tubes/balloon/valves/mask).
1 x paediatric reusable breathing circuit (tubes/balloon/valves/mask).
1 x adult reusable breathing circuit (tubes/balloon/valves/mask).
2 x soda lime absorber grains, pack/2.5 kg (depending on model supplied).
1 x spare parts/maintenance kit (air filters, tubing, O-rings).
1 x set of spare fuses, if required.
ITEMS REQUIRED, BUT NOT SUPPLIED
S0002031, Monitor,patient,portable,w/access.
OPTIONAL ACCESSORIES AVAILABLE
At extra costs additional optional features can be requested:
- A fully integrated compressor. (depending on the model supplied).
- A fully integrated active scavenging system.
- A fully integrated SpO₂ system.
- A fully integrated gas measuring system.
- Integrated end-tidal CO₂ with capnography.
ESTIMATED LIFE SPAN
8 – 10 years.
WARRANTY
Two years from shipping date.
ENVIRONMENTAL CONDITIONS
- Operating conditions: 15°C - 35°C / 95% RH.
- Storage conditions: -5°C - 40°C / 95% RH.
- Atmospheric pressure: 500 ~ 1060 hPa.
WEIGHT AND VOLUME
Weight: 137 kg.
Volume: 953 dm³.
ESTIMATED DELIVERY LEAD TIME
120 days
INSTALLATION REQUIREMENTS
Assembly and commissioning this product should be carried out by qualified technician.
TRAINING REQUIREMENTS
User training prior to utilization is recommended.
MAINTENANCE/USER REQUIREMENTS
As per user and service manuals.
RELATED PRODUCTS
S0002017, Syringe pump,with accessories.
S0002034, Infusion pump,with accessories.
S0002059, Defibrillator,basic,w/access.
S0002028, Pump,suction,surgical,1 bottle,w/access.
S0002029, Pump,suction,surgical,2 bottles,w/access.
S0746706, Laryngoscope,neonate,set.
S0746707, Laryngoscope,adult,child,set.
S0383000, Tube,endotrach,3,w/o cuff,ster,disp.
S0383010, Tube,endotrach,3.5,w/o cuff,ster,disp.
S0383020, Tube,endotrach,6.5,w/cuff,ster,disp.
S0383030, Tube,endotrach,7,w/cuff,ster,disp.
S0383040, Tube,endotrach,7.5,w/cuff,ster,disp.
S0383050, Tube,endotrach,8,w/cuff,ster,disp.
S0374010, Tube,suction,CH08,L50cm,ster,disp.
S0374015, Tube,suction,CH10,L50cm,ster,disp.
S0374025, Tube,suction,CH14,L50cm,ster,disp.
S0374030, Tube,suction,CH16,L50cm,ster,disp.
S0845003, Resuscitator,hand-oper.,neonate,set.
S0845004, Resuscitator,hand-oper.,child,set.
S0845005, Resuscitator,hand-oper.,adult,set.
COMPONENT OF A KIT
No part of a kit.
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier (if not the manufacturer) at a minimum is certified for ISO 9001 Quality management systems – Requirements.
CLASSIFICATION
Classified either under EU MDD 93/42/ECC, or under EU MDR 2017/745 as Class IIb device.
SAFETY & PRODUCT STANDARDS
- IEC 60601-1:2005 + A1:2012(E) Medical electrical equipment - Part 1: General requirements for basic safety and essential performance.
- IEC 60601-1-2:2014 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests.
- 60601-1-8 Medical electrical equipment - Part 1-8: General requirements for basic safety and essential performance - Collateral Standard: General requirements, tests, and guidance for alarm systems in medical electrical equipment and medical electrical systems.
- 60601-2-13 Medical electrical equipment — Part 2-13: Particular requirements for the safety and essential performance of anaesthetic systems.
- 80601-2-55 Medical electrical equipment — Part 2-55: Particular requirements for the basic safety and essential performance of respiratory gas monitors.
NOMENCLATURE
- GMDN code: Anaesthesia workstation, closed-circuit (34432).
- UMDNS code: Anaesthesia Systems (35373), Anaesthesia Units (10134).
Related Products