S0845048
Humidifier bottle, nonheated, reusable
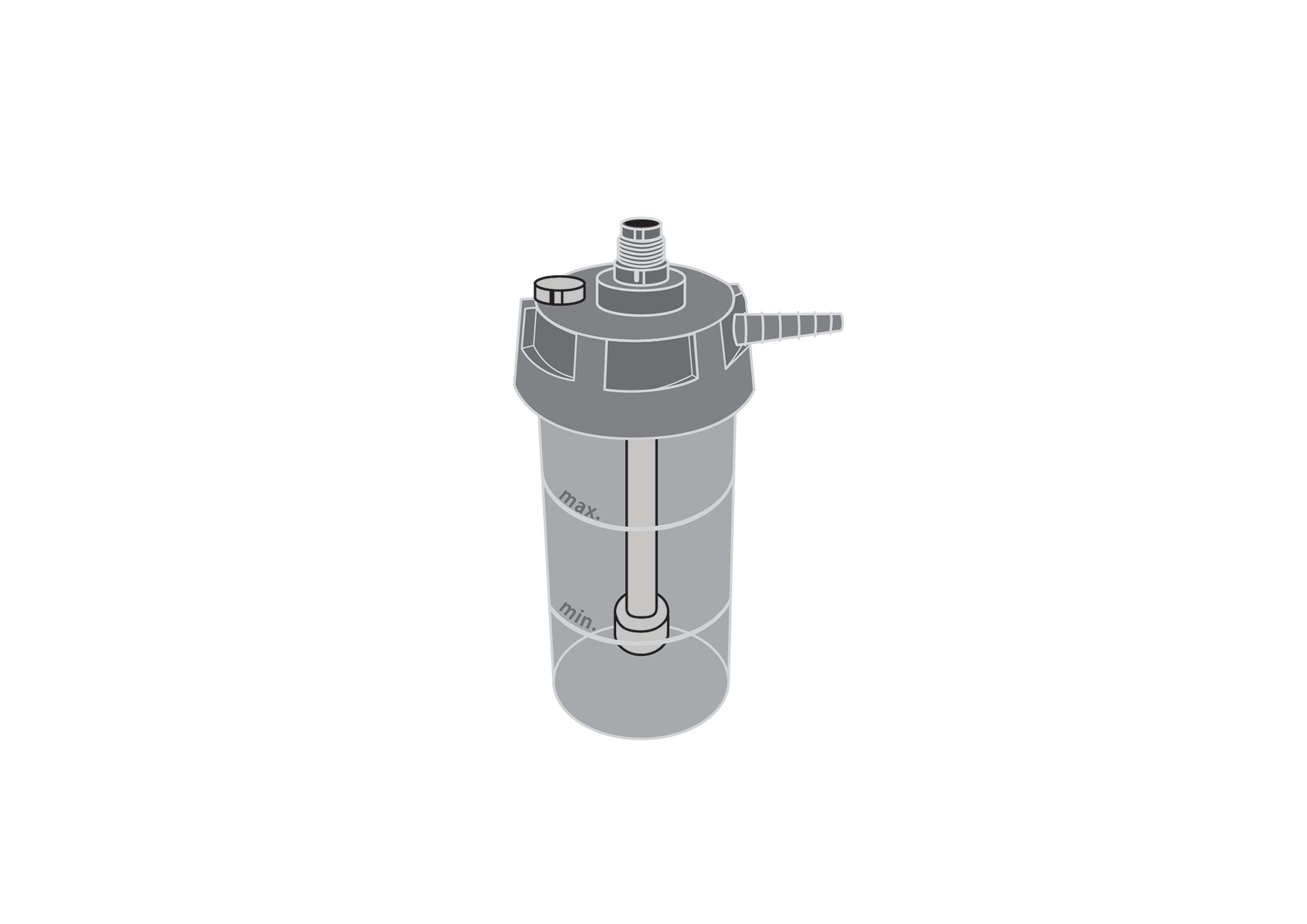
A sealed bottle of water inserted into a breathing circuit to add moisture to the breathing gases for administration to a patient. This type of humidifier does not heat the gas.
Indicative Price 9.01 USD
GENERAL DESCRIPTION
A sealed bottle of water inserted into a breathing circuit to add moisture to the breathing gases for administration to a patient. This type of humidifier does not heat the gas.
TECHNICAL SPECIFICATIONS
Non-heated, reusable humidifier for oxygen therapy and ventilation/anaesthesia inspiratory lines.
Bubble-through humidification system.
Graduated, transparent humidification bottle, shatter resistant.
Graduation shall show minimum and maximum water level.
Detachable metal or rigid durable polymer cap with gas connectors.
DISS connectors for gas inlet and outlet
All available adaptors for gas connection to be described in bid response
Autoclavable and/or disinfectable with hospital grade detergents.
Humidification chamber working volume at least 150 ml, not greater than 500 ml.
Flow rate capacity up to 15 L/min.
Safety pop-off valve incorporated
Cap and connectors made of brass/steel/other biocompatible metal or polymer certified for medical use.
Bottle and tubes made of polymer certified for medical use, shatter resistant.
ENVIRONMENTAL CONDITIONS
Operating conditions temperature: 5 to 45°C / RH: 15 to 90%
Storage conditions temperature: -20 °C to 60°C / RH: 15% - 90%
SUPPLIED WITH
1 x Spare O-ring/seal.
1 x Outlet connector for 6 mm barbed connection to oxygen tubing included.
WEIGHT AND VOLUME:
Weight: 0.146 kg (per piece, packaged)
Volume: 0.627 dm3 (per piece, packaged)
ESTIMATED LEAD TIME:
6 weeks
WARRANTY:
24 months
COMPONENTS OF A KIT
No
REGULATION & CONFORMITY REQUIREMENTS
CE mark conforming to Medical Device Directive 93/42/EEC
CE Certificate (class IIa or higher)
Or
Class 1, USA FDA
SAFETY & PRODUCT STANDARDS
Complies with the following standards:
ISO 13485:2003 Medical devices -Quality management systems -- Requirements for regulatory purposes
PACKAGING / LABELLING
Unit presentation: 1 (one)
Labelling on primary packaging must include:
- Name and/or trademark of the manufacturer
- Manufacturer's product reference
- Type of product and main characteristics
- If the packaging is not transparent, it must bear a diagram showing the essential parts of the product
- Information for particular storage conditions (temperature, pressure, light, humidity, etc.), as appropriate (or equivalent harmonised symbol)
- Information for handling, if applicable (or equivalent harmonised symbol)
Over packaging: Packaging unit
Labelling on the packaging unit: Labelling to be the same as primary packaging
Related Products