S0002019
Ventilator,medical,adult-child,w/access
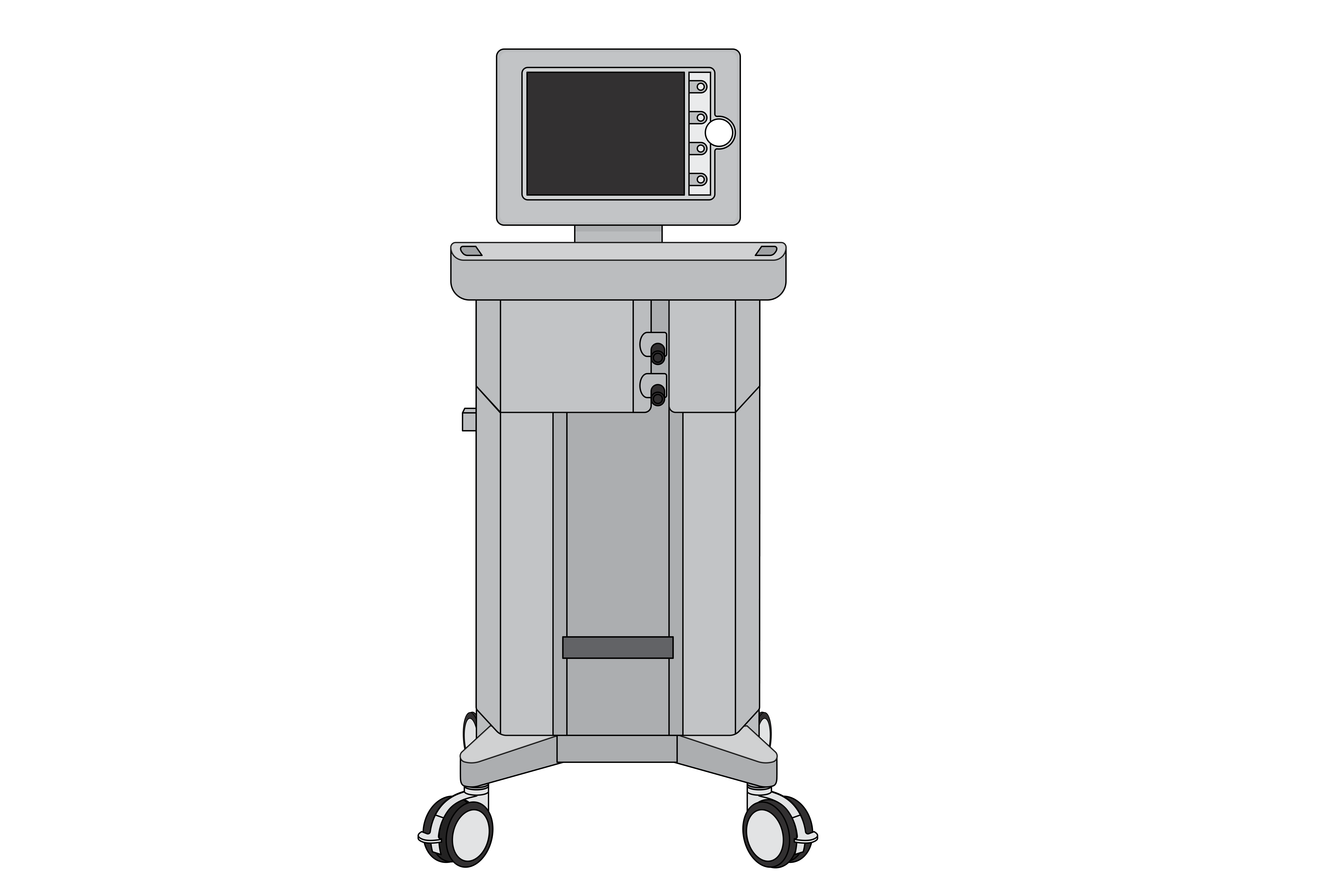
Ventilator, Intensive Care, for adult, paediatric and newborn patients. The unit is AC and battery powered, has an internal turbine for medical air and comes complete with accessories. Suitable for intubated ventilation or non-invasive (face mask) ventilation. Not suitable for ventilation while transporting patients.
Indicative Price 22,548.12 USD
GENERAL DESCRIPTION
Ventilator, Intensive Care, for adult, paediatric and neonatal patients, AC and battery powered, with accessories
IMPORTANT
Training and installation are not included with the device and must be requested separately from the supplier or manufacturer. Although these devices are designed to be life-sustaining, improper operation or maintenance can result in harm to patients and/or operators, including death. Therefore, UNICEF strongly recommends the inclusion of installation and training services for both operators and technicians, unless identical models of these devices are already in use at the intended installation site. Neither UNICEF nor the manufacturer can assume responsibility for any consequences arising from incorrect operation and/or maintenance Training and installation will have to be quoted separately and will require the number of operators and technicians to be trained and the locations where these trainings will have to take place.
NOTE:
- Oxygen concentrators cannot be used as an source for oxygen for this ventilator.
- No patient interfaces included with the device, these should be ordered separately.
For any inquiries regarding this device, please contact the UNICEF Supply Division via email at: medical-technical@unicef.org
INTENDED USE
Mechanical ventilators are life support devices that move gas (e.g., air and/or oxygen) to and from a patient's lungs. These devices may provide temporary or permanent respiration for patients who cannot breathe on their own, or who require assistance maintaining adequate ventilation because of illness, trauma, congenital defects, or the effects of drugs (e.g., anaesthetics). In most cases, mechanical ventilators are used for a short period of time (a few days to a few weeks) to deliver pressurized medical gases to the patient's lungs to support gas exchange and rest ventilatory muscles until the patient is able to breathe without mechanical assistance. Some patients, however, require permanent ventilatory support.
TECHNICAL SPECIFICATIONS
Automatic ventilator for adult, paediatric and neonatal patients.
Equipped with an internal turbine to eliminating the need for external supply of compressed air.
The unit should be mounted on a sturdy trolley with handles.
The trolley is equipped with 4 antistatic swivel castors.
At least two castors have been equipped with brakes.
Designed for frequent and easy dismount and disinfection with hospital-grade products.
Adjustable patient-circuit support arm.
Equipped with integrated oximeter for FiO₂.
Equipped with an Air/O₂ mixer.
Ability to calculate intrinsic PEEP volume, occlusion pressure (calculated manually) and inflection points.
Unit is fitted with temperature sensor.
Unit is fitted with patient circuit.
Unit is fitted with condensate collection device.
Unit is fitted with anti-bacterial filter.
Automatic compliance and leakage compensation for circuits and tubes.
Equipped with an autoclavable expiration block.
Reusable, removable and disinfectable expiration valve/flow sensor.
The unit accepts inlet gas supply pressures between 2.8 bar to 6 bar (40 to 87 psi).
Gas inlet connections are standard DISS (Diameter Index Safety Standard)
connectors.
The unit is equipped with a servo-controlled humidifier.
The humidifier has a heat exchanger which allows for temperatures between 28°C and 39°C at the Y-piece.
The humidifier temperature monitoring has an accuracy of at least ± 1°C.
The humidifier has the ability to switch between passive and active humidification.
In active mode the humidity levels the humidifier can provide are in the range of 33 mg H₂O/L to 44 mgH₂O/L.
In passive mode the humidity levels the humidifier can provide are at least 30 mg H₂O/L.
The resistance of the humidifier is no less than 0.5 cm H₂O.
The compliance of the humidifier is below 1.1 mL/cm H₂O.
The humidifier has a sterilisable humidifier chamber.
Equipped with a display (colour, flat panel ≥ 10 Inch) showing:
- Operational status.
- Set and measured parameters and values.
- Waveforms.
- Alarms/errors such as ventilation parameters, power, and system events, etc.
Equipped with a drugs nebulizer.
The unit is equipped with self-diagnosis and sensor calibration processes.
Built-in rechargeable battery, autonomy > 1 hour (standard ventilation, excluding compressor).
Automatic switch from mains to battery in case of power failure.
Automatic battery charge when mains connection is re-established.
Power requirements: units should be available for 100 and 240 V - 60 / 50 Hz.
VENTILATION MODES
The unit supports the following ventilation modes:
- Assist Control mode.
- Pressure Controlled Ventilation (PCV), pressure-controlled breaths.
- Volume Controlled Ventilation (VCV), volume-controlled breaths.
- Pressure-Regulated Volume Control (PRVC).
- Continuous Mandatory Ventilation (CMV).
- Synchronised Intermittent Mandatory Ventilation (SIMV), volume-controlled breaths, pressure-controlled breaths, and pressure support.
- Continuous Positive Airway Pressure mode (CPAP) and pressure support.
- Apnoea-backup ventilation mode.
- Spontaneous.
- (Volume targeted) Pressure controlled ventilation (APCV-TV).
- (Volume targeted) Pressure support ventilation (PSV-TV).
- Pressure Support Ventilation (PSV).
CONTROLS AND SETTINGS
The unit supports the following controls and settings:
- Positive End Expiration Pressure (PEEP): 0 - 50 cmH₂O.
- Pressure Support: 0 - 80 cmH₂O.
- Peak Inspiration Pressure: 2 - 80 cmH₂O.
- Tidal Volume: 2 - 3,000 ml.
- Respiration rate: 4 - 150 bpm.
- SIMV Respiration rate: 1 to 60 bpm.
- I/E ratio 1:10 to 4:1.
- Inspiratory Flow: 1 - 190 l/min
- Inspiratory Time: 0.036 – 9.6 sec.
- FiO₂: 21 - 100%.
- Inspiration pause time: 0 – 60% of the inspiration time.
- Inspiration rise time adjustable in four steps.
- Expiration time: 0.08 – 10.9 sec.
- Apnoea time adjustable: 5 to 60 Sec.
MONITORED AND MEASURED PARAMETERS
The below listed parameters are monitored and measured:
- I/E ratio.
- Inspiratory and expiratory times.
- Tidal Volume.
- Inspiratory and expiratory volumes.
- Minute Volume (spontaneous and mechanical).
- Respiratory rate (spontaneous and mechanical).
- Total frequency.
- Mean airway pressure.
- Peak inspiratory pressure.
- PEEP.
- FiO2 (analysed %).
- Air and O₂ supply pressures.
- Plateau Pressure.
- Resistance and compliance.
- Accuracy pressure readings equal or better than: ± 2 cm H₂O.
- Accuracy volume readings equal or better than: ± 10% or 1 ml, whichever is greater.
- Accuracy O₂ concentration readings equal or better than: ± 3%.
- Minimum of 24 hours trends storage.
- Event log which stores past 100 alarm events or events over the past 24 hrs.
- Display of compliance, resistance waveforms: Pressure – Time, Flow – Time, Volume – Time.
- Display of time loops: Pressure – Volume, Flow – Volume, Pressure – Flow.
AUDIO AND VISUAL ALARMS
The unit supports alarms for the below listed parameters and features:
- Audio-visual alarms for all measured and monitored parameters.
- Disabled alarms should be indicated on the display.
- Silenced alarms should reactivate automatically within two minutes.
- The volume of the alarm cannot be turned down to an inaudible level.
- Airway pressure (adjustable, high, low, peak pressure).
- Respiratory rate (adjustable, high).
- Tidal Volume (adjustable, high/low).
- Minute Volume (adjustable, high/low).
- FiO2 (adjustable, high/low).
- PEEP (adjustable, high/low).
- Apnoea.
- Inverse IE ratio.
- Patient circuit disconnection.
- Occlusion and/or leakage in the breathing circuit.
- Sensor failure.
- General failure to operate.
- Failure in O₂ and/or air supply.
- Low, empty or battery failure.
- AC power supply failure.
SUPPLIED WITH
Instructions for assembly, use and maintenance in English, French and Spanish.
1 x Plastic protective dustcover.
5 x neonatal, 5 x infant, and 10 x adult: autoclavable, breathing circuits (tubes/balloon/valves/mask).
4 x additional spare humidifier bottle including connection circuit.
1 x spare rechargeable battery pack.
2 x test lung, adult/paediatric.
2 x test lung, neonatal.
1 x set gas supply hoses with a length of at least 3 meters.
1 x spare parts/maintenance kit (all required: air filters, tubing, sealing/O-rings).
ITEMS REQUIRED, BUT NOT SUPPLIED
S0383000, Tube,endotrach,3,w/o cuff,ster,disp
S0383010, Tube,endotrach,3.5,w/o cuff,ster,disp
S0383020, Tube,endotrach,6.5,w/cuff,ster,disp
S0383030, Tube,endotrach,7,w/cuff,ster,disp
S0383040, Tube,endotrach,7.5,w/cuff,ster,disp
S0383050, Tube,endotrach,8,w/cuff,ster,disp
S0845215, Laryngoscope,neonate,set
S0845216, Laryngoscope,adult,child,set
S0002031, Monitor,patient,portable,w/access
Face mask (for non-invasive use)
Patient circuits
OPTIONAL ACCESSORIES AVAILABLE
At extra costs additional optional features can be requested:
- Integrated end-tidal CO₂ with capnography
- Electrical medical air compressor able to deliver medical grade air with peak output of minimum 160 Lpm. Automatically activate in the event of wall air supply loss. Allows replacement of internal filters should without removing the compressor.
ESTIMATED LIFE SPAN
More than seven years.
WARRANTY
Two years from shipping date.
ENVIRONMENTAL CONDITIONS
- Operating conditions: 10°C - 40 °C / 0% – 95% RH
- Storage conditions: -25°C - 70°C / 0% – 95% RH
- Automatic compensation up to 5,000 meter above sea level.
- Ingress protection rating: IP21
WEIGHT AND VOLUME
Weight: 85 kg.
Volume: 1,000.00 dm³.
ESTIMATED DELIVERY LEAD TIME
120 days.
INSTALLATION REQUIREMENTS
Assembly and commissioning this product should be carried out by qualified technician.
TRAINING REQUIREMENTS
Operators and technician training prior to utilization is strongly recommended.
MAINTENANCE/USER REQUIREMENTS
As per user and service manuals.
RELATED PRODUCTS
S0002017, Syringe pump,with accessories
S0002031, Monitor,patient,portable,w/access
S0002034, Infusion pump,with accessories
S0002059, Defibrillator,basic,w/access
S0383000, Tube,endotrach,3,w/o cuff,ster,disp
S0383010, Tube,endotrach,3.5,w/o cuff,ster,disp
S0383020, Tube,endotrach,6.5,w/cuff,ster,disp
S0383030, Tube,endotrach,7,w/cuff,ster,disp
S0383040, Tube,endotrach,7.5,w/cuff,ster,disp
S0383050, Tube,endotrach,8,w/cuff,ster,disp
S0845014, Pulse oximeter,portable,w/access
S0845017, Pulse oximeter,handheld, incl.resp. rate
S0845019, Pulse oximeter, tabletop
S0845154, Laryngoscope,neonate,set
S0845155, Laryngoscope,adult,child,set
COMPONENT OF A KIT
No part of a kit.
QUALITY MANAGEMENT SYSTEM
- Manufacturer is certified for ISO 13485 Medical devices - Quality management systems - Requirements for regulatory purposes.
- Supplier (if not the manufacturer) at a minimum is certified for ISO 9001 Quality management systems – Requirements.
CLASSIFICATION
Classified either under EU MDD 93/42/ECC, or under EU MDR 2017/745 as Class IIb device.
SAFETY & PRODUCT STANDARDS
- IEC 60601-1:2005 + A1:2012(E) Medical electrical equipment - Part 1: General requirements for basic safety and essential performance.
- IEC 60601-1-2:2014 Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral standard: Electromagnetic compatibility - Requirements and tests.
- IEC 60601-1-6:2010 Medical electrical equipment - Part 1-6: General requirements for basic safety and essential performance - Collateral standard: Usability.
- IEC 60601-1-9:2007 Medical electrical equipment - Part 1-9: General requirements for basic safety and essential performance - Collateral Standard: Requirements for environmentally conscious design.
- ISO 80601-2-12:2011 Medical electrical equipment - Part 2-12: Particular requirements for basic safety and essential performance of critical care ventilators.
- ISO 80601-2-55:2018 Medical electrical equipment - Part 2-55: Particular requirements for the basic safety and essential performance of respiratory gas monitors.
- ISO 80601-2-74:2017 Medical electrical equipment - Part 2-74: Particular requirements for basic safety and essential performance of respiratory humidifying equipment.
- ISO 5356-1:2004 Anaesthetic and respiratory equipment -- Conical connectors -- Part 1: Cones and sockets.
- ISO 5367:2014 Anaesthetic and respiratory equipment -- Breathing sets and connectors.
- ISO 14971:2007 Medical devices -- Application of risk management to medical devices.
- ISO 15001:2010 Anaesthetic and respiratory equipment -- Compatibility with oxygen.
- ISO 21969:2009 High-pressure flexible connections for use with medical gas systems.
- ISO 27427:2013 Anaesthetic and respiratory equipment -- Nebulizing systems and components.
NOMENCLATURE
- GMDN code: Neonatal/adult intensive-care ventilator (42411).
- UMDNS code: Ventilators, Intensive Care (17429).
Related Products